INTRODUCTION
Renal replacement therapies are frequently used in the intensive care unit (ICU) in the context of acute kidney injury (AKI). Severe AKI is a common complication in critically ill patients, occurring in approximately 50% of all patients requiring admission in the ICU.1 Severe AKI is associated with up to 60% hospital mortality.2 These patients usually require renal replacement therapies (RRT) which, in this setting, include conventional intermittent hemodialysis (IHD), continuous renal replacement therapies (CRRT), prolonged intermittent renal replacement therapies (PIRRT) and peritoneal dialysis (PD).
AKI in the severely ill patient generally appears as a component of a multiple organ disfunction in the context of a systemic insult such as shock or a major surgery. These patients frequently require a high volume of intravenous medications leading to hyperhydration in the setting of oliguria.
Additionally, they are frequently hemodynamically unstable requiring high doses of catecholamines, leading to a significant difficulty in fluid removal.3 The acute renal replacement therapies (ARRTs) should not deteriorate cardiovascular instability, that would increase the risk of end-organ damage and potentially decrease the chances of renal recovery.4 The optimal RRT modality for patients with AKI is still controverse.
The choice of RRT modality in the ICU is frequently limited by the centre capacity and experience in addition to the patient’s clinical features (e.g., hemodynamic stability, hemorrhagic risk, cerebral oedema, poisoning, need of procedures that require interruption of the treatment).
The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for AKI (2012) suggest using CRRT, rather than standard intermitente RRT, for hemodynamically unstable patients (Grade 2B), but in its rationale they concluded that sustained low-efficiency dialysis (SLED) may also be tolerated in hemodynamically unstable patients with AKI.5
The most studied prolonged intermittent RRT (PIRRT) is SLED since it is the treatment more often performed. Our aim is to review the characteristics of the SLED technique in the ICU setting and discuss when it should be started, comparing its advantages and disadvantages over IHD and CRRT.
THE SLED TECHNIQUE
SLED is an intermittent treatment (at least three times per week) that provides RRT for an extended period of time with lower solute clearances and lower ultrafiltration rates (UFR) comparing with IHD. It overlaps characteristics of both CRRT and IHD hence the use of the term “hybrid technique”.6 SLED aims to combine the hemodynamic stability of CRRT and the efficiency of IHD.7 The modality classically semipermeable membrane - diffuse clearance) which is named SLED.
Prescription can also be performed with hemofiltration (where the application of a hydrostatic pressure into the blood compartment, leads to solute drag with the ultrafiltered water through a semipermeable membrane resulting in the clearance of small and middle-sized molecules - convective clearance) or hemodiafiltration that combines both diffuse and convective clearances known as SLED-F. In SLED-F, besides the use of dialysis fluid, infusion of a replacement fluid is needed. The effluent fluid is the sum of the dialysate solution, the replacement fluid, and the excessed fluid removed. The use of convective clearance promotes a better removal of middle weight molecules than diffusive clearance, namely pro-inflammatory cytokines, with potential outcome benefits. However, there is insufficient data to support a recommendation in this regard.8
Machinery
The devices used in SLED are the same dialysis machines routinely used in IHD. The machinery can be single pass or batch machines. In single pass machines the dialysate is produced on-line from reverse osmosis purified water. In batch machines the dialysate is generated from prepackaged salts and sterile water6 and stored within the dialysis machine. Therefore, these machines do not require the usual infrastructure (multilocal water supply or waste removal), and nowadays all the necessary set is portable.
The dialysers used in SLED are also the same typically used for IHD (high-flux and high-efficiency). However, dialyser selection will depend on the selected modality (SLED vs SLED-F) and patient’s characteristics.
Less efficient dialysers should be used in the first few sessions to prevent disequilibrium syndrome. In SLED-F, the membrane should have a high hydraulic permeability (ultrafiltration coefficient ≥ 20 mL/h/mmHg), high solute permeability (Beta-2 microglobulin clearance ≥ 20 mL/min and vitamin B12 ≥ 80 mL/min) and large surface of exchange (1.5-2.1 m2).9
Prescription
Session length
The session length may vary between 6 to 18 hours a day. Session length should be individualized according to patient’s needs. The two major determinants of session length are patient’s volume status and hemodynamical stability. One way to estimate the session length is to divide the ultrafiltration goal (the volume that we want to remove on SLED session) by the maximum ultrafiltration rate tolerated by the patient. It may be necessary to evaluate the initial patient response to ultrafiltration and adjust the prescription if needed. Logistical reasons may also influence session length, namely the schedule of the patients’ procedures and of the available human resources.
As an intermittent modality, SLED has potential benefits for patient’s ICU routine and management. The timing and duration of therapy can be adaptable to eventual diagnostic and therapeutic procedures that would result in CRRT interruptions and compromise of the dialysis dose provided. SLED is also a technique that can be done at night, which allows patient mobility during the day, enhancing physical recovery.
Dialysate flow rate
The dialysate flow rate (Qd) ranges from 100 to 300 mL/min. A higher flow rate is usually used when a greater solute clearance is necessary as when patients present with severe acidosis, hyperkalaemia or poisoning. The dialysate flow rate is also adjusted in accordance with the session length. In sessions with a duration superior to 8 hours, the Qd is decreased to 100 or 200 mL/min, while sessions with duration inferior to 8 hours, the Qd usually is of 300 mL/min.6
Blood flow rate
Blood flow rate (Qb) usually varies between 200-300 mL/min, depending on the quality of the vascular access. Higher blood flows prevent clotting, allowing lower doses of anticoagulation. Higher blood flow does not worsen hemodynamic instability since the inflow and outflow through the vascular access are the same. At most, the returning blood will be slightly more concentrated due to some eventual ultrafiltration. Still, a high Qb can promote a rapid small solute clearance and produce osmolality changes that can affect hemodynamics.
Although solute clearance may be increased with greater Qb, it is limited by the saturation of the dialysate, particularly at low dialysate flow rates, as it is illustrated in Fig. 1.11 The flattening curves represents the Qd value above which increasing Qb does not improve urea clearance.
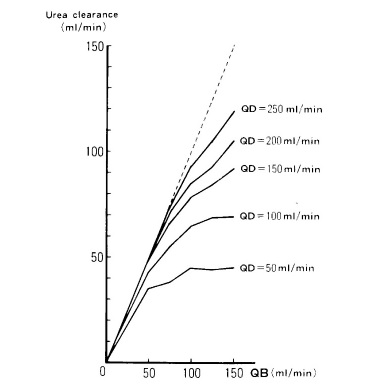
Figure 1 Relationship between blood flow rate (Qb), dialysate flow rate (Qd) and urea clearance during SLED
Data from: Kudoh Y, Iimura O. Slow continuous hemodialysis - new therapy for acute renal failure in critically ill patients - Part 1. Theoretical consideration and new technique.
In addition to having a hemodynamic impact, osmolality changes induced by rapid removal of urea (and other small solutes) can cause water shifts that can lead to cerebral oedema and neurological symptoms that compose the dialysis disequilibrium syndrome (DDS).9
Despite being more common in dialysis induction of patients with end-stage renal disease, it can also occur in patients with severe AKI and markedly elevated blood urea levels. It can be prevented by using smaller dialysers and by reducing Qb and Qd. Performing isolated ultrafiltration before or after dialysis to limit urea clearance when there is significant volume overload, may also be considered. Although SLED and CRRT lead to slower solute clearance compared to IHD, DDS can also rarely occur with these modalities.12
Infusion flow rate - in SLED-F
There is little data in the literature about the specificities of SLED-F prescription. Most authors have standardized protocols, with infusion rates that vary between ~33 and 100 mL/min).8,13-15Prefilter is frequently used to minimize coagulation problems.
It has to be considered that in a hemodiafiltration prescription, the rate of replacement fluid infusion must be one third of the blood flow in a post-dilution modality and half the blood flow in pre-dilution, to minimize the risk of clotting.10 Modern dialysis machines have auto-substitution systems that optimise infusion flows, while maintaining transmembrane pressures within margins of effectiveness and attempting to avoid hemoconcentration. This is the preferred substitution modality in many ICU units where SLED-F is used.
Ultrafiltration rate
The first challenge in defining the required total ultrafiltration is to understand the degree of volume overload. Possible approaches to assess volume overload include: physical examination (presence of tissue oedema, weight, capillary refill); evaluation of patient fluid balance (total fluid intake minus total fluid loss); hemodynamic parameters (blood pressure, heart rate, pulse pressure, stroke volume variation); blood oxygenation, chest radiograph, lung ultrasound; echocardiogram to check for inferior vena cava inspiratory variability; bioimpedance.16 There is no single parameter that gives us the volume status of the patient. An integration of several of these parameters should always be made. Frequent assessment of patient fluid balance is necessary to adjust the needed ultrafiltration.
Net ultrafiltration rate is the volume of fluid removed per unit of time. It excludes the replacement volume from the ultrafiltrate when a convective modality is used and reflects the true volume of fluid removed from the patient. An optimal net ultrafiltration rate should allow time for vascular refilling to occur, in order to minimise the risk of hemodynamic instability. The movement of fluid from the interstitial and intracellular compartments should balance the fluid removed from ultrafiltration. Vascular refiling not only depends on UF rates but also on plasma oncotic pressure, fluid overload, dialysate sodium concentration, body size and properties of capillary endothelial barrier.
When vascular refilling is not fast enough, intravascular hypovolemia takes place, which will result in decreased preload and cardiac output, hypotension and decreased organ perfusion. Additionally, as already mentioned, clearance of solutes results in a reduction in plasma tonicity promoting volume shift from the intravascular to the interstitial space, consequently worsening intravascular hypovolemia and impairing ultrafiltration. Other patient-related factors may further deteriorate cardiovascular reserve during ultrafiltration, such as heart failure, diabetes and the presence of reduced vasomotor tone, making it harder to establish an adequate net ultrafiltration rate.16
A safe net ultrafiltration rate in patients with acute kidney injury under SLED therapy is not described in the literature yet. Data is only available in the field of CRRT, in which a moderate ultrafiltration rate (1.01-1.75 mL/kg/h) in critical ill patients is associated with lower mortality. However, in patients with fluid overload and refractory hypoxemia with acute respiratory distress or severe left ventricular failure, a higher ultrafiltration rate may be necessary for a short period of time. Low net ultrafiltration rates (<1.01 mL/kg/h) are associated with prolonged exposure to fluid overload, organ oedema and may increase the duration of mechanical ventilation. High net ultrafiltration rates (>1.75 mL/kg/h) can lead to myocardial ischemia, cardiac stunning, regional cardiac wall motion abnormalities and increase the risk of cardiac arrythmias. It can also lead to hypoperfusion of other organs (brain, kidneys and gastrointestinal tract).16
We can only extrapolate that the “moderate ultrafiltration” used in CRRT would probably be safe in SLED. Clinical trials are needed to address this issue.
Dosing
The KDIGO guidelines for AKI (2012) recommends delivering a Kt/V of 3.9 per week or a Kt/V of 1.3 in a thrice-weekly prescription, when using IHD or extended RRT in AKI.5 Nevertheless, in critical care, where the course of the disease, the fluid status and the metabolic requirements are dynamic, the use of Kt/V ratio to quantify the dose of delivered dialysis is rarely used. In practice, dialysis dose is adjusted according to clinical course and daily laboratory analysis. Nonetheless, it is relatively easy to reach those targets in an extended RRT session.
The Hannover Dialysis Outcome17 study compared 2 doses of SLED in a total of 156 ICU patients: a standard-dialysis arm dosed to maintain plasma urea levels between 120-150 mg/dL and an intensified-dialysis arm dosed to maintain plasma urea levels below 90 mg/dL. Mortality and kidney recovery at 28 days were similar between the 2 groups. Kt/V was not calculated. These findings indicate that increasing the dose of SLED might neither reduce mortality nor improve renal recovery in critically ill patients with AKI.
Another study, from the VA/NIH Acute Renal Failure Trial Network,18 that included 1124 patients, also compared the intensity of renal support in critically ill patients with acute kidney injury. In the less-intensive therapy strategy, HDI and SLED were provided 3 times per week and CRRT was prescribed to provide a total effluent flow rate of 20 mL per kilogram per hour. In the intensive therapy strategy HDI and SLED were provided 6 times per week and CRRT was prescribed to provide a total effluent flow rate of 35 mL per kilogram per hour.
Once again, there were no significant differences between groups in mortality (51.5% in the less-intensive therapy and 53.6% in the intensive therapy), rate of renal recovery and duration of renal replacement therapy.
Dialysate composition
The dialysate for SLED can be generated on-line, be provided as prepackaged fluid, or mixed from prepackaged salts prior to the treatment.
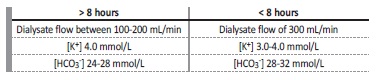
The dialysate composition prescription varies with session length and patient’s requirements.6 If the session duration is superior to 8 hours, the dialysate usually contains potassium of 4 mmol/L and bicarbonate of 24-28 mmol/L. If the session duration is less than 8 hours, the dialysate generally contains potassium of 3-4 mmol/L and bicarbonate of 28-32 mmol/L, as represented in Table 1. Calcium in the dialysate may vary between 1.5 to 2.5 mmol/L.6 It may be necessary to adjust the dialysate composition according to intra-dialytic or postdialysis laboratory values.
Anticoagulation
One of the potential benefits of SLED and IHD over CRRT is the reduced need of anticoagulation. It may be a safer option when there is considerable risk of complications from the two most common anticoagulation options: unfractionated heparin (UFH) and regional citrate anticoagulation (RCA). Saline flushes and increasing Qb may be sufficient to avoid the use of anticoagulation in selected cases.
Nevertheless, there is a significant incidence of circuit clotting without anticoagulation (26%-46%).19
Unfractionated heparin
UFH is the most frequent anticoagulation used in SLED. The usual loading dose is 1000-2000 UI bolus, followed by an infusion of 500- 1000 UI/h, to keep an activated partial thromboplastin time (aPTT) 1.5 times above the upper limit of the normal range. UFH has a short half-life (90 minutes, increasing to up to 3 hours in renal insufficiency)20 and is reversible with protamine. The therapeutic index is narrow and is associated with risk of hemorrhage and heparin-induced thrombocytopenia (HIT).
Regional citrate anticoagulation
In RCA, citrate chelates calcium, an essential component of the coagulation cascade.9 Citrate-calcium complex dissociates in the circulation and is metabolized to bicarbonate in the liver and, to a lesser extent, in the skeletal muscle and in the kidney.21
RCA prescription has greater complexity than heparin anticoagulation, involving a calcium infusion and close monitoring, which implies a higher involvement of the medical and nursing staff. It is also more expensive than anticoagulation with UFH. Citrate accumulation is the most frequent complication of RCA. Citrate metabolism can be slowed when liver function is impaired leading to citrate accumulation and metabolic and electrolyte complications, such as metabolic acidosis and low systemic ionized calcium levels. Other possible metabolic and electrolyte disturbances include metabolic alkalosis, hypernatremia or hyponatremia, hypercalcemia and hypermagnesemia or hypomagnesemia.
These complications are uncommon when an RCA protocol is used.22 Several RCA protocols have been published. In the most frequente method, the citrate is infused upstream from the dialyzer, a zero-calcium dialysate is used (to minimize the citrate dose required) and calcium is replaced in the venous return line.23 Other possible method is to maintain calcium in the dialysate and not reinfuse calcium systemically. In this method, although reported as safe by several studies, serious complications have been reported (namely, severe hypocalcemia and cardiac arrest).24
When correctly used, RCA may have efficient and safety advantages over UFH in SLED. A retrospective study from Germany19 with 282 critically ill patients compared different outcomes with citrate and/or UFH anticoagulation in SLED modality. The RCA protocol consisted in an infusion of 30% trisodium citrate in the arterial line and calcium chloride-dihydrate into de venous line. The dialysate contained 1.0 mmol/L of calcium. The monitorization consisted of: evaluation of post-dialyser ionized calcium concentration (target range 0.35-0.45 mmol/L) measured 30-60 minutes after SLED initiation and re-evaluated when demanded; and blood gas analysis every 2 hours (pH, Na+, K+, Ca2+, bicarbonate). In total, 976 SLED sessions with heparin and 808 with citrate were analysed. In-hospital mortality did not significantly differ between the 2 groups. Citrate anticoagulation was superior to heparin in preventing circuit clotting (5% with citrate vs 10% with heparin). Three patients in the heparin group died from severe bleed complications vs none in the citrate group. Metabolic complications (metabolic acidosis and electrolyte disturbances) and hypotension occurred more frequently in citrate, although not significantly.
Low-molecular weight heparin
Low-molecular weight heparin (LMWH) has a longer half-life, greater bioavailability, lower risk of bleeding (due to a lower impact on platelet function) and lower incidence of HIT than UFH. Its major disadvantage over UFH is that it is not readily reversible with protamine.9 The half-life of LMWH is extended in renal failure and one administration at the beginning of dialysis usually suffices for up to 5 hours. Enoxaparin 40 mg can be given as a loading dose, followed by 10 to 40 mg every 6 hours if needed.24
Prostacyclin
Another alternative anticoagulant for the SLED circuit is prostacyclin. Prostacyclin is as product of human endothelium with antiaggregating effects, at low doses.25 At higher doses, it has vasodilatory effects. It has a short half-life and has shown a lower bleeding risk than heparin.24 In an Italian study25 with 35 ICU patients treated with SLED, the use of prostacyclin for anticoagulation was analysed in 185 SLED sessions. Therapeutic intervention for hypotension (increasing vasopressors and/or fluid reposition) was required in 45 of 185 SLED sessions and 2 sessions were interrupted due to refractory hypotension. Although the study concluded that prostacyclin is a safe and effective anticoagulant agent for SLED, it does not appear to be the best option in critically ill patients.
Vascular access
The vascular access for SLED is usually a nontunnelled or tunnelled catheter in a central vein. Outer catheter diameter usually varies between 12-16 French. A larger diameter is preferred to allow a greater blood flow rate and, consequently, a higher dose of RRT. However, a larger catheter diameter may increase the risk of vein stenosis and this must be taken into account. The preferred site for CVC placement is the right internal jugular vein (RIJV), followed by the femoral veins, left internal jugular vein (LIJV), and lastly the subclavian veins.5 The RIJV having a more direct line to the cavoatrial junction is associated with a higher blood flow and less complications (vein stenosis, kinking, obstruction).
The use of femoral vein limits patient mobility and consequently may impair patient’s physical recovery. One of its advantages is that there is no risk of pneumothorax or hemothorax and is a useful approach in patients with acute pulmonary oedema. This placement site has the higher risk of infection and is associated with higher recirculation rates.
The subclavian site is associated with high incidence of insertion related complications and central vein stenosis which compromises a future permanent access, if needed. The recommended catheter length varies depending on the vein and patient’s size. In the upper location, the catheter tip should be at the junction between de superior vena cava and the atrium.9 For femoral catheters, the tip should be in the inferior vena cava. Generally, for RIJV is recommended a length of 15cm, for LIJG 15 to 20 cm and for femoral veins a length of at least 24 cm.26
Arteriovenous fistula (AVF) or graft (AVG) of chronic hemodialysis patients can be used for SLED although they are less recommended due to the risk of accidental needle dislodgement, potentially leading to bleeding or injury to the AVF/AVG.7 When several SLED sessions are expected, placement of a dialysis catheter is recommended. In patients on hemodialysis with a history of central vein stenosis, use of AVF or AVG may be the better option, though they need a close clinical monitoring.
Complications
SLED is associated with both technique and clinical complications. Technique complications are common to IHD and CRRT modalities and include vascular access malfunctions, hematomas, pneumothorax/hemothorax, catheter kinking, circuit clotting, line-catheter disconnection and air embolism. Clinical complications include a wide range of complications, from coagulation disturbances, infection and sepsis, allergic reactions, cardiovascular and hemodynamic instability, electrolyte abnormalities and nutrient losses.27
The more relatively common complications associated with SLED are described below.
Hypotension
Although ultrafiltration in SLED is generally well tolerated, hypotension can occur. In the literature, only a minority of patients (0%-7%) had to discontinue extended dialysis treatment due to intractable hypotension.6
Temporary increase of inotropes may occur but it is not frequent.8
Hypophosphatemia
Longer treatments are associated with more frequent electrolyte abnormalities, specially when high-flux dialysers are used. Hypophosphatemia is one of the most common abnormalities, often leading to the need of phosphate replacement (intravenously or by adding phosphorus to the dialysate).4 Hypophosphatemia can result in respiratory muscle weakness, increase the risk of arrhythmia and is associated with longer ICU and hospital stay.28
Amino acid losses
Amino acid losses in SLED are significant. Protein supplementation
of more 0.2 g/kg/day should be administered on treatment days.4,6
Intoxications
IHD is the most commonly used therapy for poisoning (usually with small, non-protein bound agents such as toxic alcohols, lithium, valproate, salicylate, theophylline). There are only a few studies in the literature about SLED in poisoning compared with other RRT modalities (IHD and CRRT).29 Sustained low-efficiency diafiltration (SLED-f) has been reportedly used for poisoning treatment.30 Theoretically, the addition of convection increases the clearance of middle molecules and could be beneficial in some cases. SLED and CRRT may have a role in selected patients, by preventing a new increase in plasma poison concentration following an intermittent treatment, limiting the rebound effect of some toxins (like in lithium toxicity). SLED may be also employed after IHD to minimize the risk of toxin rebound. The hemodynamical safe profile of SLED and CRRT may not be superior to IHD in some poisonings when no net ultrafiltration is required.
When poison removal is urgent, SLED and CRRT are not the treatments of choice, unless ultrafiltration is required in an unstable patient.29,31,32
Drug dosage
There are considerable differences between SLED, IHD and CRRT in the rates of drug removal.
Drug dosing in SLED is challenging because of high heterogenicity of treatment prescription. Therefore, there is substantial risk of underdosing essential drugs, like antibiotics.
Drug clearance during RRT is affected by various factors33:
- Mechanism of solute removal: Diffusion is the most effective method in removing small molecules (<500-1000 Daltons). Transport by convection is independent of solute concentration throughout the membrane and the rate of transport is independent of molecule size, removing larger size molecules better than diffusion.34
- Filter membrane permeability: High-flux dialysers have larger pore sizes and allow the removal of molecules with a size up to 20 000 Daltons (vs low-flux dialysers that only allow clearance of molecules up to 500 Daltons).
- Blood and dialysate rates: Increasing Qb and Qd will normally improve the clearance of solutes.
- Residual renal function: Renal function recovery should be monitored, as it contributes to drug excretion.
- Drug properties: molecular size, charge, protein binding, volume of distribution, water or lipid solubility.
- Timing of drug administration: for drugs administered intermittently, administration in the start or during SLED will affect overall drug exposure. For drugs administered continuously, that are removed by SLED, modification of infusion rate should be considered.
Various dosing recommendations frequently provide separate dosing “during-SLED” and “non-SLED days”33 or propose a constant baseline dose plus an additional dose given at the end of SLED session.35
The pharmacodynamic profile of an antibiotic, either if its antimicrobial activity is concentration-dependent (e.g., aminoglycosides and quinolones) or time-dependent (e.g., beta lactams) should also be taken into account when using SLED.33,34 For example, an aminoglycoside can be given before a SLED session permitting the achievement of a high Cmax, followed by clearance with SLED to reduce potential toxicity.35 Drug levels should be performed whenever possible.
WHEN TO START SLED
The general indications for initiating SLED are the same for other RRT modalities: chronic kidney disease stage 5D, complicated severe AKI and intoxications with dialysable drugs.36 The precise timing to start RRT in these situations, goes beyondthe scope of this review.
When a decision to start RRT in the ICU is taken, the clinician may choose, according to the availability and personnel and centre experience, to start IHD, SLED, CCRT or PD. In developed countries, PD is rarely used for RRT naïve patients, and so will not be addressed here.
The most frequent indication for SLED modality is AKI requiring RRT in a mild hemodynamically unstable patient that cannot tolerate conventional IHD. IHD is more suitable for patients who require rapid removal of dialysable substances and that are hemodynamically stable, like in severe hyperkalaemia and in intoxications by toxins that require fast removal.
CRRT seems to be a better option than SLED and IHD when overt hemodynamic instability is present and in cases of AKI that have concomitant increased intracranial pressure or brain oedema.5,37
CRRT allows a slower fluid removal and a slower control of solute concentration, avoiding significative fluctuations in solute concentration and fluid shifts.5 In fact, some particularities of critically ill patients explain why continuous techniques are the most used modalities in the ICU setting. These patients often require large amounts of fluid (from parental nutrition, medications or initial hemodynamic resuscitation), which determine the need of a high daily UF that is more easily obtained by a slow continuous UF.27 Nevertheless, SLED can be an option in hemodynamically unstable patients that do not require high doses of inotropes and vasopressor agents. The patient’s response to ultrafiltration should be monitored in order to avoid episodes of hypotension, which can lead to renal ischemia and lower the likelihood of renal recovery. Comparison between SLED and CRRT in terms of hemodynamic stability, ultrafiltration, mortality, kidney recovery, hospital/UCI length of stay (LOS) and metabolic control is discussed below.
A controverse topic is the use of RRT in ICU setting in an attempt to modulate pro-inflammatory cytokine in septic AKI. Cytokine removal by extracorporeal blood purification has been proposed as a possible therapeutic choice to improve outcomes in critically ill patients. Many clinicians choose hemofiltration or hemodiafiltration in septic patients in an attempt to remove middle molecules (inflammatory cytokines) with convective clearance.38 Although clinical benefits have been suggested, studies have not yet shown a clearly advantage in clinical outcomes of hemofiltration over hemodialysis. Furthermore, high cut-off (HCO) filters have been safely used to increase the removal of cytokines from the circulation in septic AKI patients. A recent study (2019)39 compared the use of HCO vs traditional high-flux dialysers in sustained low-efficiency diafiltration (SLED-f). HCO-SLED-f provided significant IL-8 and TNF-α reductions. Nevertheless, the degree of all cytokine reductions did not show significant differences between the 2 groups. There were significantly higher total albumin losses in HCOSLED-f group, which can further worsen the patient nutritional status.
No significant difference in intra-dialytic blood pressure during both treatments was found and finally no differences in the hard outcomes. High-intensity RRT has also been tried with no clear advantages but clearly complicating the antibiotic dosage management and possibly jeopardizing adequate therapeutic levels.38
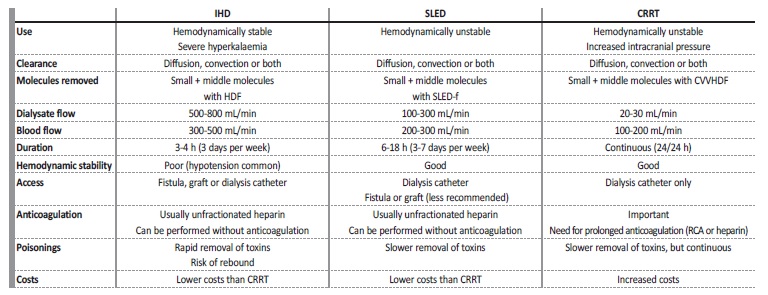
Table 2 summarizes the main features of each modality of RRT prescribed in the ICU.
COMPARISON OF PIRRT AND CRRT IN CRITICALLY ILL PATIENTS
There are several comparative studies between PIRRT and CRRT modalities published in the literature. The summary of the most importante are presented in Table 3 and some of the most relevant are commented below. A 2015 meta-analysis (Zhang et al)40 of 17 studies comparing PIRRT and CRRT, 16 of which are included in the Table 3,15,41-55showed no difference in mortality rates between the 2 modalities. In the observational studies,15,41,42,44-49,55 PIRRT was associated with a lower mortality risk, although these studies are potentially subject of selection bias. There were no significant differences in fluid removal, episodes of vasopressor escalation, efficacy, ICU LOS and recovery of kidney function. In terms of costs, the results showed that they were lower with PIRRT.44,52,53 A 2017 meta-analysis (Kovacs et al),56 that included 18 studies, corroborated the findings of Zhang et al meta-analysis.
Table 3 Studies comparing SLED and CRRT modalities.
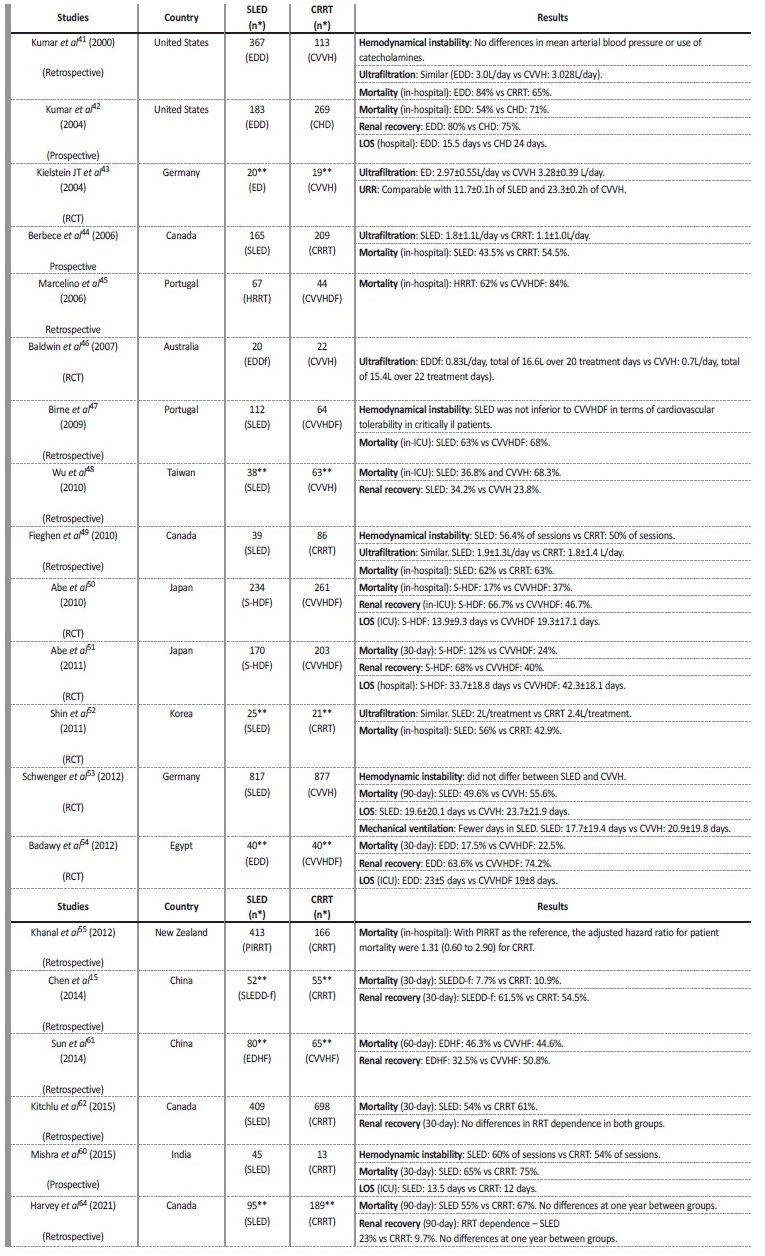
CHD - continuous hemodialysis; CRRT - continuous renal replacement therapy; CVVH - continuous venovenous hemofiltration; CVVHDF - continuous venovenous hemodiafiltration; ED - extended dialysis; EDD - extended daily dialysis; EDDf - extended daily diafiltration; EDHF - extended daily hemofiltration; HRRT - hybrid renal replacement technique; LOS - length of stay; PIRRT - prolonged intermitente renal replacement therapy; RCT - randomized control trial; S-HDF - sustained hemodiafiltration; SLED - sustained low-efficiency dialysis; SLEDD-f - sustained low-efficiency daily hemodiafiltration; UF - ultrafiltration; URR - urea reduction rate. * Number of days of treatment; ** Number of patients.
Another meta-analysis from 2017 (Nash et al),57 tried to compare outcomes between CRRT, IHD and PIRRT modalities among critically ill patients. A significatively advantage for any RRT modality on mortality or dialysis dependence was not observed. Studies comparing hospital or ICU LOS for PIRRT versus CRRT showed that patients usually had briefer LOS with PIRRT modality, though these results were based on only five studies.42,50,51,53,54The authors raise a highly relevant point, that in common practice patients frequently transition between different modalities and, in many studies, it is only analysed patients on their initial modality. This fact is not considered in multiple studies.
A 2021 meta-analysis (Ye et al),58 which included 30 RCTs, also compared different RRT modalities (IHD, PIRRT, CRRT and PD) in critically ill patients with AKI. The RCTs used to compare CRRT and PIRRT are represented in the Table 3.50-54SLED, especially SLED-f, was associated with decreased mortality compared with CRRT, but with a low certainty evidence. SLED was also correlated with an increased renal recovery compared with CRRT and IHD, again with these conclusions limited by imprecision and low certainty evidence. SLED may also be associated with shorter hospital LOS and days of mechanical ventilation.
The more rapid mobilization of patients with SLED modality, may be responsible for the shorter hospital stays and more rapid convalescence.
Another 2021 meta-analysis (Dalbhi et al),59 concluded that there was no major advantage using CRRT compared with PIRRT in hemodynamically unstable patients. They reported no significantly diferences between the 2 modalities in terms of mortality rate, renal recovery, dialysis dependence, UCI LOS and fluid removal rate. Efficacy between PIRRT and CRRT was also similar. This meta-analysis included all of the Ye et al meta-analysis, and a few others, all represented in the Table 3 (6 RCTs43,50,51,53,54,60and 5 prospective cohort studies.15,42,48,61,62).
There are several studies, also represented in Table 3, comparing anticoagulation between PIRRT and CRRT. In respect to UFH overall, they conclude that a lower dose of heparin is used in PIRRT. A lower dose of heparin implies a lower risk of bleeding, which is significant in the critically ill patient. However, in CRRT, regional citrate anticoagulation is the anticoagulation currently recommended.5 Therefore, the potential benefit of using PIRRT to minimize the dose of heparina was lost. Nevertheless, when RCA is not recommended, PIRRT without circuit anticoagulation is associated with a lower risk of circuit coagulation than CRRT and can be a good option in these situations.
Another study worth mentioning is a retrospective Spanish study63 that included 54 patients that received SLED as first therapeutic option or after receiving CRRT or IHD. The authors observed that the majority of SLED treatments were indicated after poorly tolerated IHD, secondly, as a “step-down” between CRRT and IHD and less frequently, as firstline treatment in critically ill patients, concluding that SLED is used as an intermediate step between CRRT and IHD or for patients that do not have hemodynamical stability for IHD.
In summary, SLED has been shown to be non-inferior to CRRT on multiple studies and meta-analysis. This modality presents a favourable cardiovascular profile and similar efficacy, mortality and kidney recovery when compared to CRRT. Nevertheless, there is an importante selection bias in most studies, with the most hemodynamic unstable patients being frequently not included in the analysis. Moreover, most studies are small sample-sized and there is a great heterogeneity between CRRT and especially PIRRT prescriptions, which limit study comparison and makes it difficult to draw definitive conclusions.64
CONCLUSION
SLED is the most common PIRRT technique, combining advantages of both IHD and CRRT. It can be used as a first choice in the mild hemodynamic unstable patient or as a bridge between CRRT and HDI. Selection of the best treatment should be individualised for patient characteristics, centre experience and logistical capacity of the ICU.
Many potential benefits are seen with the SLED modality. Compared with classic IHD, it allows a greater hemodynamic stability and a slower UF rate. Compared to CRRT, due to the fact that it is it na intermittent RRT, SLED allows early patient mobilization (and, consequently, a faster physical recovery) and the realization of necessary therapeutic and diagnostic procedures without technique interruption (and, consequently, without compromising RRT dose). Interestingly, some studies suggest SLED may be associated with better renal recovery and even hard outcomes such as patient mortality, than CRRT.
SLED may also be a better option when there is considerable risk of complications from heparin or RCA since it can be performed without anticoagulation with a reduced risk of circuit coagulation. Additionally, SLED is associated with lower human resources and financial costs than CRRT.
One major concern of using SLED is the lack of evidence regarding the optimal dose and timing of drug prescription, especially antibiotics. Close monitoring and prescription adjustments to SLED may be necessary to ensure therapeutic drug levels. In conclusion, SLED provide a safe and cost-effective RRT to the critically ill patient in the ICU setting. High-quality RCTs are needed to clarify the role of SLED in critically ill AKI patients.