INTRODUCTION
Accidental or intentional poisoning and drug overdose are a significant source of morbidity, mortality and health care expenditure worldwide.1 A poison is any substance, including any drug, that can harm a living organism regardless of intention. Poisoning generally implies that damage results from exposure to pharmaceuticals, illicit drugs or chemicals.
Correct management of poisoning begins with a thorough evaluation, recognition that poisoning has occurred, identification of agent(s) involved and assessment of severity. Treatment includes supportive care, prevention of poison absorption and, when appropriate, the use of antidotes or interventions to enhance the elimination of poison, as urine alkalinization or extracorporeal treatment, especially when natural elimination mechanisms are impaired. The intervention of a nephrologist or intensivist is often necessary, to correct electrolyte or acid-base disturbances, manage kidney or other organ dysfunctions and evaluate the need for extracorporeal treatment (ECTR).2
Currently there is a lack of randomized control trials comparing extracorporeal versus support treatment, due to the relative rarity of poisonings treated with extracorporeal treatment, the low mortality from most poisons, the heterogeneity of poisoned patients and the ethical obstacles when measuring ECTR versus placebo.3-5 However since 2010, a multidisciplinary group named EXtracorporeal TReatments In Poisoning (EXTRIP), has been reviewing and publishing evidence-based recommendations, grading the available evidence and establishing guidelines to standardize the use of extracorporeal detoxification in poisoned patients.3,5
The aim of this narrative review is to describe the available extracorporeal modalities that can be used for poisoned patients, reflecting on their main indications and limitations and provide a practical view on the management of the most common poisons found in clinical practice.
METHODS
A non-systematic search of the PubMed database for English-language articles using the keywords “extracorporeal treatment”, “hemodialysis” and “poisoning” was performed to construct this narrative review. Case reports and case series deemed relevant to the authors were also included for specific poisons. Additionally, review papers were hand-searched to identify potentially pertinent articles.
A MATTER OF TOXICOKINETICS: WHEN TO USE EXTRACORPOREAL TREATMENT
The removal efficacy of poisons from plasma by ECTR depends highly on the poison characteristics. To be removed effectively, it must be water soluble, have low molecular weight (MW), low protein binding and low volume of distribution (< 1 L/kg), as ECTR only clears substances from the intravascular compartment.4,5This partly explains why poison removal is time sensitive - in the first hours after an acute poisoning a higher proportion of poison is in the intravascular compartment.
Volume distribution (Vd) is one of the greatest determinants of efficacy of extracorporeal removal of poisons and is defined by the theoretical dispersion of the substance in the body, calculated by amount of drug in the body concentration of drug in the plasma.
It is determined by the lipophilicity of the substance (lipophilic substances have a higher Vd) and is affected by the patient’s characteristics and comorbidities (e.g., obesity, extracellular fluid volume, cardiac output, renal function, age and sex).5
It is unclear when to use ECTR as it is usually reserved for a small number of patients. Its use may be justified if the ingested quantity is associated with severe, life-threatening toxicity, particularly if natural removal mechanisms are impaired, if there is a high likelihood of permanent disability, if the patient presents clinical deterioration (hypotension, metabolic acidosis, respiratory depression or life-threatening dysrhythmias) or after supportive measures fail.6
AVAILABLE MODALITIES FOR ECTR: WHICH MODALITY OF ECTR SHOULD WE USE?
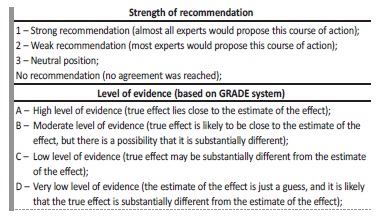
ECTR are a heterogeneous group of treatments that promote removal of both endogenous and exogenous poisons. Different modalities are available and an understanding of their indications and limitations are key to choose the most appropriate ECTR for each clinical situation. Table 1 summarizes the main differences between the most common used modalities.
Table 1 Modalities used in extracorporeal treatment of poisons
iHD: intermittent hemodialysis; CRRT: continuous renal replacement therapy; iHF: intermittent hemofiltration; MW: molecular weight; iHDF: intermittent hemodiafiltration; HP: hemoperfusion; TPE: therapeutic plasma exchange
Intermittent hemodialysis. Intermittent hemodialysis (HD) removes poisons through diffusion across a semi-permeable membrane down a concentration gradient from blood to dialysate. The efficacy of treatment depends on the membrane surface area, time of treatment, blood and dialysate flow rates and characteristics of the poison itself.7 It is considered the treatment of choice in almost all substances suitable for extracorporeal removal, because of its wide availability, fast substance removal and low MW of most common poisons.4,5Besides its effectiveness in removing poisons, it also allows correction of fluid balance, electrolytes and metabolic abnormalities.
Intermittent hemofiltration. In hemofiltration, both poison and solvent are removed by convection and replaced by a physiological solution. Intermittent hemodiafiltration combines convection and diffusion requiring both dialysate and replacement fluid. These convective-based techniques have similar removal properties as HD, considering Vd and protein binding. These are particularly useful in the removal of high MW poisons. However, as most common poisons have low MW, as stated before, hemofiltration and hemodiafiltration do not seem to have any advantage over HD.4,5
Hemoperfusion. Hemoperfusion uses charcoal or resin-containing cartridges to which the poison is adsorbed. It has historically been used for poison removal (e.g., theophylline and barbiturates). Nonetheless, its use has declined over the years, probably reflecting improvements and availability in HD technique, cost of the technique, fast cartridge saturation and complications of hemoperfusion (e.g., clotting, hypocalcemia, hypophosphatemia). As of today, hemoperfusion remains the preferred treatment for paraquat poisoning (often in combination with HD).8
Continuous renal replacement therapy. Continuous renal replacement therapies comprise continuous venovenous hemodialysis, continuous venovenous hemofiltration and continuous venovenous hemodiafiltration. These techniques are mainly used in intensive care units and are useful in managing acute kidney disease patients, especially if fluid overload is present and hemodynamic stability is a limiting factor for high-efficiency intermittent techniques.
All principles regarding diffusion and convection mentioned for intermittent techniques are applied to continuous techniques.
The main difference, which is also its main limitation, is that both blood and effluent flows are lower comparing to intermittent techniques and therefore clearance will be lower over a similar period of time. This difference allows it to overcome the main limitation of intermittent techniques regarding hemodynamic factors, as said before, but also to maintain poison removal for longer periods, avoiding toxic rebound (sudden increase in plasma poison concentration following an intermittent treatment) as seen characteristically in slow distribution poisons, as lithium.4
Therapeutic plasma exchange. Therapeutic plasma exchange involves the separation of plasma from the cellular components of blood either by centrifugation or filtration. The role in poison removal is reserved to specific situations like cisplatin overdose, iatrogenic poisoning with monoclonal antibodies or intoxication with Amanita phalloides (a highly poisonous mushroom), but evidence is modest.4,8,9
Others. Peritoneal dialysis is rarely used for poison removal as its clearance rate is low. There are some reports considering the use of extracorporeal liver assist devices for removal of proteinbound poisons (like diltiazem, phenytoin and theophylline), but the efficacy and availability is lower and the cost is higher than the ECTR mentioned above.6 These remain occasionally useful in cases of poison-induced hepatotoxicity.5 These techniques will not be reviewed in this paper.
Beyond poison removal. Besides being used for poison removal, ECTR are also increasingly used for supportive care in poisoning caused by substances not amenable for removal. This is particularly relevant in intoxications by opioids and other sedative hypnotic agents causing shock, sympathomimetics causing rhabdomyolysis and acute kidney injury, and severe acetaminophen poisoning, resulting in multiorgan failure. In these cases, continuous renal replacement therapy is potentially helpful, considering the hemodynamic limitations of intermitente techniques relevant in these situations.8
ECTR FOR INDIVIDUAL POISONS: IN WHICH POISONINGS SHOULD WE CONSIDER ECTR?
Considering the pharmacokinetics needed for an effective removal by ECTR, only a small number of poisons benefit from these techniques.
Poisons in which extracorporeal removal might be useful and should be considered are described in the following paragraphs. Lithium. Lithium is the first line therapy for mood disorders even though it has a narrow therapeutic index. Toxicity presentation is heterogeneous and it differs depending on whether the intoxication is acute or chronic, due to plasma lithium concentration and the duration of exposure to supratherapeutic concentrations. Acute intoxications are caused by an acute ingestion (e.g., in a suicide attempt).
Chronic intoxications occur when lithium intake exceeds its elimination on a chronic basis, usually over several weeks (e.g., decline in kidney function or drug interaction). The risk of neurotoxicity (with ataxia, myoclonus, confusion, convulsions or coma) is lowest with acute poisoning and highest with chronic poisoning, as lithium distributes to
the intracellular space in the central nervous system. Symptoms may also be gastrointestinal, cardiac and renal.10 Lithium is readily dialyzable (with HD as the treatment of choice) because it has a low MW (7 Da), insignificant protein-binding and a low Vd (0.7-0.9 L/kg). A fast reduction in lithium concentration by HD prevents accumulation in the brain (toxic compartment) and/or establishes a favorable concentration gradient, facilitating the diffusion of lithium into the plasma (nontoxic compartment).10,11 Nonetheless, intracellular lithium concentration falls slowly because of the slow movement between cells and plasma (slower than ECTR removal from the plasma) and a rebound may be expected 6 to 8 hours after the end of HD. This may also happen when ongoing absorption from extended-release formulations is occurring. A rebound in lithium concentration may restart treatment with an ECTR, but few patients (if any) exhibit clinical deterioration due to the rebound.10,11 With this in mind, continuous renal replacement therapy may be helpful to avoid this effect and should be considered.
Formal indications to start ECTR are not set in stone but a sérum lithium level > 4.0 mmol/L is a common indication, especially if kidney function is impaired. If the patient presents with symptoms (especially central nervous system dysfunction with decreased level of consciousness or seizures) or life-threatening arrhythmias, HD should be started despite serum levels. It should be continued until serum levels are < 1.0 mmol/L and clinical improvement is observed.11
Salicylates. The most common source of intoxication by salicylates is acetylsalicylic acid (aspirin) and it can be acute (e.g., acute ingestion in a suicide attempt) or chronic (e.g., chronic consumption in a patient with pre-existing kidney disease). Acetylsalicylic acid undergoes rapid hydrolysis to salicylate in the gastrointestinal tract, liver and bloodstream and it causes toxicity through multiple mechanisms.2
Overdose in adults is characterized by respiratory alkalosis (through stimulation of the respiratory center in medulla leading to hyperventilation) and metabolic acidosis (through lactic acid formation from interruption of oxidative phosphorylation), leading to neurological symptoms.
Salicylates have a low MW (38-180 Da) and low Vd (0.2-0.5 L/kg). At therapeutic level, 90% of salicylates are protein-bound, but the bound percentage decreases as total concentration increases (around 30% in overdose).12 Salicylates metabolization and elimination is mainly hepatic, however, toxic levels lead to saturation of hepatic detoxication and it becomes dependent of kidney excretion.
Treatment is based on aggressive volume resuscitation, until euvolemia is achieved. Oral activated charcoal may be useful if used 1 to 2 hours after ingestion. It is also important to alkalinize both blood (decreasing salicylates concentration in central nervous system) and urine (increasing excretion of salicylates). Oral bicarbonate should be avoided (as it might enhance gastrointestinal absorption), as well as acetazolamide (it alkalinizes urine by inhibition of bicarbonate reabsorption, leading to salicylate movement into the brain).13Electrolytes such as potassium and calcium should be closely monitored. Although charcoal hemoperfusion is very effective in removing salicylates, HD is the preferred method since it is easier to perform and corrects metabolic acidosis and electrolyte disturbances more efficiently.2 Main indications to start HD are a salicylates level > 100 mg/dL regardless of symptoms, lower thresholds (90 mg/dL) if kidney impaired function is overserved or irrespective of serum concentration if the patient presents with central nervous system depression or salicylate-induced pulmonary edema (non-cardiogenic) with new-onset hypoxemia.12 Tinnitus or other hearing disturbances can also be present.2 HD should be performed until salicylate level is < 19 mg/dL or clinical improvement is observed. The main goal is to reverse acidosis and alkalosis to prevent respiratory collapse.
Metformin. Metformin is the first line therapy for type 2 diabetes, as described in the most recent American Diabetes Association guidelines.14 Its efficacy is obtained by the inhibition of neoglucogenesis, facilitation of cellular glucose uptake and reduction of insulin resistance.15 Unfortunately, its use is linked with the risk of developing metformin associated lactic acidosis (MALA). Metformin increases plasma lactate by inhibiting mitochondrial respiration, especially in the liver, responsible for lactate elimination.15 This is more likely to happen when metformin is in toxic levels or in cases of renal dysfunction (even in normal doses), since its excretion is performed exclusively by the kidney (contraindicated use if estimated GFR is ≤30 mL/min/1.73 m2).2 Intoxication results in metabolic acidosis, shock and multi-organ failure. Even though MALA mortality rate may be as high as 50%, the incidence is relatively low (<10 cases for 100 000 patient-year).2,16
Treatment is primarily supportive with hemodynamic stabilization and correction of hydro-electrolytic disturbances, although the most effective and preferential treatment is HD. Metformin is moderately dialyzable as it has 165 Da, negligible protein binding and a Vd 1-5 L/kg, which is its main limitation. HD is recommended when lactate levels are high (> 20 mmol/L), leading to severe acidosis (pH <7.0), or if supportive therapy fails.15 If kidney or liver function are impaired, the patient presents with shock or decreased level of consciousness, a lower threshold for initiation of HD may be applied. HD should be suspended once lactate level is <3 mmol/L, pH >7.35 and the patient is clinically stable.15
Toxic Alcohols. A patient history of alcohol consumption is often lacking, leading to a presumptive diagnosis relying on the possible exposure in association with the presented symptoms, physical findings and blood chemistry abnormalities. Delayed treatment is associated with worse outcomes including irreversible organ damage and death, so early recognition is key to start treatment promptly.
a) Methanol intoxication occurs within 6 to 24 hours after ingestion or inhalation of windshield washer fluid or fuel-line antifreeze, generally in a suicide attempt.2 It is metabolized in the liver by alcohol dehydrogenase (ADH) and aldehyde dehydrogenase into formaldehyde and formic acid, respectively, the latter responsible for toxicity.17 It can also present kidney and respiratory excretion.
Treatment is mainly supportive and comprises antidotal use of ethanol (competitive subtract for ADH) and fomepizole (ADH inhibitor), to delay or prevent metabolism to their toxic metabolites, but not to remove them.8 Antidotes should be used if osmolal gap is > 10 mOsm/kg or blood level is >20 mg/dL. HD is very effective in removing methanol, since it is a small molecule (32 Da) with low protein binding and a Vd 0.6-0.8 L/kg, as well as its metabolites. Nonetheless, its use is reserved for patients with symptoms (e.g., coma, seizures and visual impairment), severe metabolic acidosis or anion gap >24 mmol/L, kidney dysfunction or high serum concentration of methanol (> 50 mg/dL in the absence of fomepizole or 70 mg/dL if it was used). HD may be suspended when methanol concentrations are <20 mg/dL and a clinical improvement is observed.18
b) Ethylene glycol is a compound of antifreeze and windshield washer solutions.2 It is converted by ADH and aldehyde dehydrogenase to glycolic acid, responsible for metabolic acidosis and oxalate, causing organ dysfunction through crystal precipitation.8
Same treatment principles and indications are applied as described in methanol, concerning the use of ADH competitive inhibitors. HD is helpful, as it is has low MW (62 Da), high water solubility and low Vd and it should be started when the patient presents deteriorating clinical condition, with depression of central nervous system, severe metabolic acidosis, acute kidney injury, pulmonary and cerebral edema, when serum levels are > 50 mg/dL in case fomepizole was not given or 300 mg/dL after fomepizole administration.17 Absorption in the gastrointestinal tract is fast, therefore gastrointestinal decontamination is rarely helpful. The same applies to methanol.
c) Isopropanol is used in the manufacturing of acetone and glycerin, used in hand sanitizers or rubbing alcohol and intoxication happens after ingestion or inhalation of these products.2 It is metabolized by ADH to acetone. Lethal doses for adults are as small as 100 mL. Signs of intoxication appear within 1 hour of ingestion and include gastrointestinal and neurological symptoms (e.g., confusion, stupor and coma). It can also lead to respiratory dysfunction, cardiovascular collapse, acute pancreatitis or hypotension, the latter being the strongest predictor of mortality.19
In the contrary to the previous described alcohols, there is no benefit in inhibiting ADH (as acetone is less toxic than isopropanol). HD should be started if isopropanol levels exceed 40 mg/dL and central nervous system depression, renal failure or hypotension are present, but these indications are controversial and most cases are resolved with supportive measures.2,19
d) Diethylene glycol intoxication is rare (typical of developing countries without regulatory oversight) and can occur after the ingestion of brake fluids or other industrial products.2 It can cause gastrointestinal symptoms, central or peripheral neuropathy and acute kidney disease. Fomepizole is recommended and depending on the magnitude of kidney injury HD may be necessary.17
e) Propylene glycol intoxication may happen with high dose infusion of lorazepam or diazepam at the hospital or after ingestion of antifreeze products, but its prevalence is low. Toxicity is not severe and generally resolves with vigorous hydration. If significant lactic acidosis is present, HD might be useful.17
Carbamazepine. Carbamazepine is used in the treatment of bipolar disease, neuropathic pain and seizures. Normally it is metabolized by the liver and eliminated by the kidney. Toxicity generally occurs with serum levels > 4 mg/dL. Treatment is based in supportive care with airway protection, treatment of seizures with benzodiazepines and correction of hypotension, if needed. Gastrointestinal decontamination with activated charcoal may be useful if used 1 to 2 hours after intoxication.20 HD is reserved to severe intoxications with neurological symptoms (e.g., altered mental status, refractory seizures and movement disorders), as well as respiratory depression and cardiovascular toxicity with life-threatening dysrhythmias.20 It is a small molecule (MW 236 Da) and although it has around 75% binding to proteins (even in toxic levels), is highly lipophilic with high Vd (0.8-1.4 L/kg), which makes it moderately dialyzable.2,20 Hemoperfusion is equally effective in removing carbamazepine but it is more expensive and difficult to perform. Cessation of HD is indicated when clinical improvement is observed or carbamazepine concentration is < 1 mg/dL.20
Paracetamol. Paracetamol is widely used as antipyretic and analgesic and it is the most common pharmacological agent involved in intoxications.2 It has small MW (151 Da), 25% of protein binding and a Vd of 0.8-1.0 L/kg. It is mainly metabolized by the liver (90%), a small percentage by the kidneys and the rest by cytochrome P450 in N-acetylp-benzoquinone imine (NAPQI). NAPQI is a toxic substance that binds with glutathione to form nontoxic metabolites excreted in the urine. When toxic doses of paracetamol are taken, NAPQI levels rise and exceed the available glutathione leading to mitochondrial dysfunction and toxicity, especially acute liver failure. In large doses, it can also result in encephalopathy and lactic acidosis.
Treatment generally includes gastrointestinal decontamination and antidotal therapy: N-acetylcysteine (NAC). In most cases, these therapeutic measures resolve the intoxication, and no further treatment is required. HD is reserved for cases of severe poisoning, and it is recommended if NAC is not administered and serum concentration is > 100 mg/dL or if the patient presents with altered mental status, metabolic acidosis or elevated lactate and a serum concentration > 70 mg/dL. If NAC is administered and the patient presents with the symptoms described above and the serum level is > 90 mg/dL, HD is also recommended. It should be performed until clinical improvement is observed.21
Valproic acid. Valproic acid is used in the treatment of partial and generalized seizures and bipolar disease. At therapeutic level it is 90% protein bound, but this percentage drops in overdose, making extracorporeal removal possible, as well as having a low MW and a low Vd. Poisoning leads to confusion, lethargy, nausea and vomiting, tachycardia, hypotension and electrolyte disturbances. ECTR can be considered when severe poisoning is present (serum levels > 130 mg/dL) or if the patient presents shock, cerebral edema, respiratory depression or coma. Hemoperfusion can be used, however, HD is preferred because it also reverses acidosis and corrects electrolyte disturbances.
ECTR should be continued until clinical improvement is apparent or serum concentration is between 5 and 10 mg/dL. Limited evidence exists supporting the clinical efficacy of L-carnitine as an antidote.22
Cisplatin. Cisplatin has been used as a neoplastic agent. Although it is very effective in many tumors, it has neuro-, nephron-, myelo- and ototoxicity. Despite all precautions, cisplatin overdose may occur by accident. Other than prevention, the only way to treat cisplatin overdose is by removing the drug from the plasma.23
HD can remove free cisplatin, but after administration cisplatin binds to the plasma proteins very quickly and therefore its efficacy is limited. Therapeutic plasma exchange substantially removes plasma and protein-bound fraction of cisplatin and should be performed as soon as possible as a crucial part of the treatment. Chemoprotection (e.g., sodium thiosulfate) can help avoid therapy-induced side effects and should be used early after detection of overdose.23,24
Others. Table 2 summarizes all recommendations, including poisonings mentioned above, as well as less common poisonings (atenolol, baclofen, barbiturates, gabapentin, phenytoin, thallium and theophylline), describing briefly their main characteristics, available modalities and main indications to start and cease ECTR.
STRENGTH OF RECOMMENDATION AND GRADE OF EVIDENCETable 2 32 Table 3.
Table 2 Recommendations for ECTR in selected poisonings.24,25,26,28,29,30,31
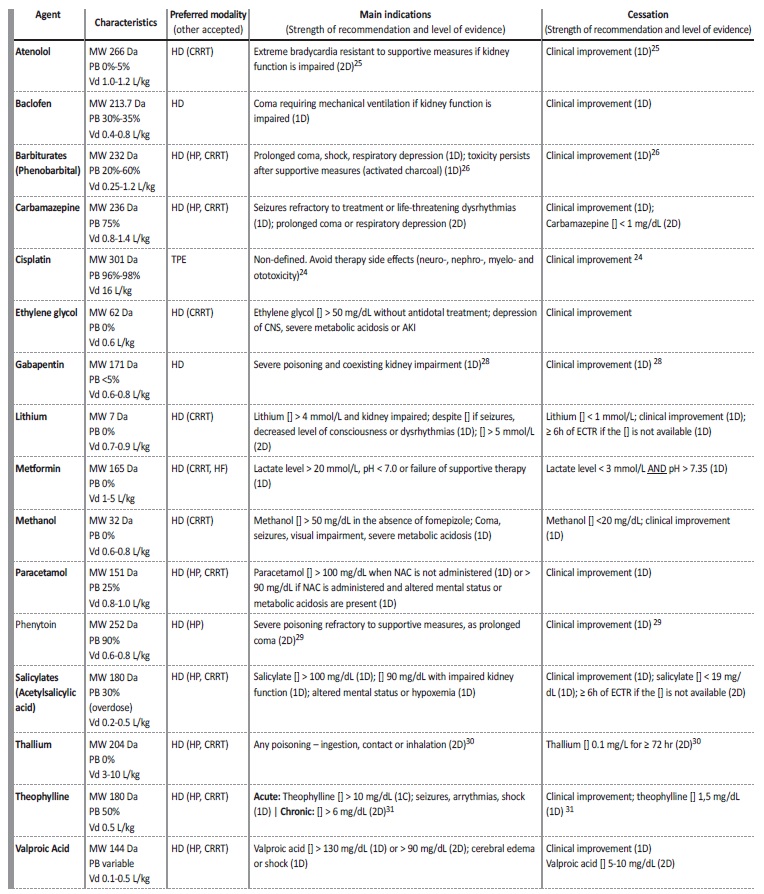
Serum concentration; NAC - N-acetylcysteine; MW - molecular weight; PB - protein binding; Vd - volume of distribution; HD - intermittent hemodialysis; HP - hemoperfusion; CRRT - continuous renal replacement therapy,TPE - therapeutic plasma exchange
Not amenable to ECTR removal:
a) β-blockers. As stated before, some β-blockers like atenolol are removed by ETCR, although others such as propranolol, timolol and metoprolol are not, considering their molecule characteristics. (1D)25
b) Tricyclic antidepressants. ECTRs are not likely to have any clinical benefit in poisoning by tricyclic antidepressants as amitriptyline and its use is not recommended.33 Some case reports defend the use of therapeutic plasma exchange but evidence is modest.34
c) Digoxin. Digoxin has a very effective antidote - digoxin imune Fab and a very high Vd (6.1 ± 2.6 L/kg) so ECTR are not useful in overdose and its use is not recommended (1D).35
d) Isoniazid. Isoniazid poisoning is more frequent in parts of the world where tuberculosis is prevalent. Treatment should focus on supportive care and pyridoxine administration, therefore, ECTR are not recommended.36
e) Calcium channel blockers. It was considered that the risks and costs associated with ECTR surpassed any potential benefit in calcium channel blockers poisoning like amlodipine, diltiazem and verapamil. (1D)37
f) Methotrexate. An expected benefit from ECTR is very limited and in rare circumstances. Although it might accelerate elimination of plasma methotrexate, its use is not supported as addition to standard care, as an alternative to glucarpidase (methotrexate antidote) or as an addition to glucarpidase in most clinical contexts.27,38
CONCLUSION
Although supportive care is the mainstay in the management of poisoned patients, ECTR are undoubtedly important as a treatment option in these cases, with HD being favored in all the most common poisons.
Evidence regarding ECTR use in poisoned patients is still limited, but workgroups such as EXTRIP are improving evidence quality with systematic reviews of the literature available, raising awareness for clinicians, and improving health care to poisoned patients.