INTRODUCTION
Acute kidney injury (AKI) is life-threatening condition characterized by a rapid decrease in renal function.1 AKI is frequent in hospitalized patients and its incidence is higher in critically ill patients, in whom the leading cause of AKI is sepsis, with frequent requirement for renal replacement therapy (RRT).2-4 AKI has been associated with longer hospital stays, in-hospital mortality, progression to chronic kidney disease and long-term mortality.5-7
Septic-AKI patients have specific characteristics than distinguish them from non-septic AKI, that implicates more non-renal organ failure and requirement of vasopressors and mechanical ventilation. The prognosis is also worse, with prolonged length of hospital stays and higher short-term mortality.3-8
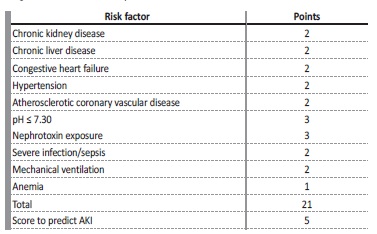
Over the past decades, a few models examined clinical risk factors for the development of AKI in the Intensive Care Unit (ICU) population, although with small populations studied. A recent multicenter study from Malhotra et al9 prospectively analyzed a population of 1300 patients admitted in an ICU and concluded that chronic kidney disease (CKD), chronic liver disease, congestive heart failure, hypertension, atherosclerotic coronary vascular disease, pH<7.3, nephrotoxin exposure, sepsis, mechanical ventilation and anemia were identified as independent predictors of AKI.9 This study was also able to develop a risk model, based on chronic comorbidities and acute events and define an optimal cutoff value of ≥ 5 points as an increased risk for development of AKI (Table 1). Although the optimal timing for the initiation of RRT in AKI is not consensual, there are no available models that can reliably predict the need for RRT once AKI is established.10-18
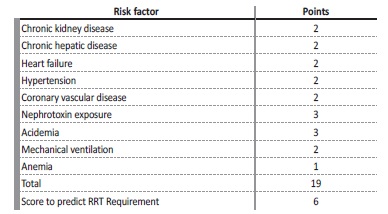
We hypothesized that it was possible to adapt this risk score (Table 2), based on routinely available clinical variables, to predict the risk of requiring RRT once AKI has been established. For this purpose, we cross-examined data from a retrospective study in which we studied a cohort of critically ill patients with septic-AKI.19,20
METHODS
This is cross-examination of a retrospective analysis, conducted in a single center, that included septic-AKI patients admitted to the Division of Intensive Medicine of the Centro Hospitalar Universitário Lisboa Norte (CHULN), Portugal, between January 2008 and December 2014.
This study was approved by the Ethical Committee in agreement with institutional guidelines. Due to the retrospective and non-interventional nature of the study, informed consent was waived by the Ethical Committee.
Participants
Eligible patients were selected as adult patients (≥18 years of age) with a diagnosis of sepsis at admission to the Division of Intensive Medicine who developed AKI within the first week of ICU hospitalization.
Exclusion criteria comprised CKD patients on renal replacement therapy, patients who underwent RRT one week prior to admission to the ICU and patients who were discharged or died less than two days after ICU admission.
Variables and outcomes
Patient variables were collected from individual clinical records. The protocol for all patients in this ICU includes daily determination of serum creatinine (SCr) and hourly urine output (UO).
We analyzed several clinical variables including patient demographic characteristics (age, gender, ethnicity, body weight and height), comorbidities (hypertension, diabetes mellitus, coronary vascular disease, congestive heart failure, CKD, chronic hepatic disease, neoplasia), main diagnosis on admission (medical versus surgical nature), source of infection, laboratory values at admission (serum hemoglobin, serum albumin, SCr and pH analysis), disease severity according to the Simplified Acute Physiologic Score (SAPS) II21 as determined by the worst variables documented throughout the first 24 hours of ICU admission, fluid balance during ICU admission, mechanical ventilation, vasopressor use and requirement for renal replacement therapy. The primary outcome was RRT requirement.
Definitions
We used Kidney Disease Improving Global Outcomes (KDIGO) classification according to both SCr and UO to define AKI.21 Pre-admission SCr (SCr within the previous three months) was considered as baseline value. When unavailable, baseline SCr was estimated from the MDRD equation21 accepting the lower limit of a normal baseline GFR of 75 mL/min/1.73 m2.
Sepsis was diagnosed according to the third international consensus definitions as an acute change in total Sequential Organ Failure Assessment(SOFA) score ≥2 points consequent to the infection.22 Diabetes mellitus was diagnosed according to the American Diabetes Association criteria23 and hypertension was diagnosed according to the seventh report of the Joint National Committee.24 Chronic Kidney Disease was classified according to KDIGO classification.25 Anemia was defined as hemoglobin level below 12 g/dL. Acidemia was defined as a serum pH level above 7.35. Previous diagnosis of coronary vascular disease, neoplasia, chronic heart failure and chronic hepatic disease of any cause was also documented (clinical records were considered sufficient for the confirmation of these diagnosis).
The Renal Replacement Therapy Risk Score was adapted from the AKI risk prediction score proposed by Malhotra et al.9 This Risk Score was calculated as is discriminated on Table 2. This risk score comprises the following variables: chronic kidney disease, chronic liver disease, heart failure, hypertension, coronary vascular disease, nephrotoxin exposure, acidemia, mechanical ventilation, anemia. By adding the variables, the total score can range from a minimum of 0 to a maximum of 19 points. We excluded patients the variable “severe infection/ sepsis” from the original score because the population only included septic patients.
Statistical methods
We described categorical variables as the total number and percentage for each category, whereas continuous variables were described as the mean ± standard deviation. Normally distributed continuous variables were compared with the Student’s t-test, nonnormally distributed continuous variables were compared with the Mann-Whitney U test and categorical variables were compared with the chi-square test.
Only variables that significantly differed between RRT requirement and no need for RRT groups were used in the univariate and multivariate analysis using the logistic r egression method. Univariate analysis was performed in all variables to determine statistically significant factors that may predict RRT requirement in septic-AKI patient. Only variables with a significant statistical difference were included in the multivariate analysis using the Cox logistic regression method. To prevent collinearity, variables which were part of the RRT risk score were not assessed in the multivariate analysis.
The discriminatory ability for this Risk Score to predict the need for RRT requirement in septic-AKI patients was determined using the receiver operating characteristic (ROC) curve. A cut-off value was defined as that with the highest validity.
Data were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was defined as a p-value <0.05. Statistical analysis was performed with the statistical software package SPSS for windows (version 21.0).
RESULTS
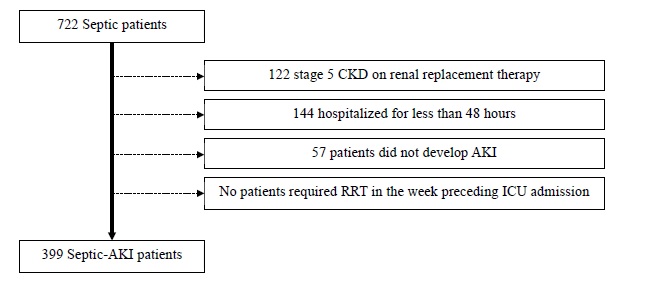
We identified 722 septic patients. Of these, 323 were excluded as followed: 122 had stage 5 CKD on renal replacement therapy, 144 had been hospitalized for less than 48 hours and 57 patients did not develop AKI during ICU stay. No patients required renal replacement therapy in the week preceding ICU admission (Fig. 1). A final cohort of 399 patients was studied.
The characteristics of this population have been previously described in a study in which we assess the utility of the neutrophils, lymphocytes and platelets ratio as a predictor of mortality in septic-AKI patients.19 Demographic variables, clinical and laboratory characteristics are described in Table 3.
Table 3 Patients’ baseline characteristics and according to renal replacement therapy (RRT) requirement
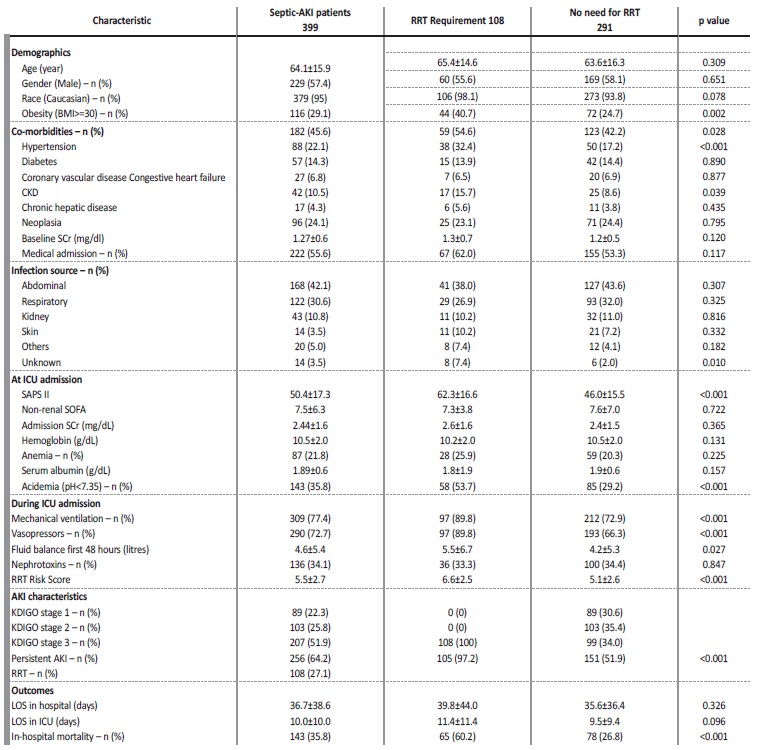
BMI - body mass index; CKD - chronic kidney disease, SCR - serum creatinine; ICU - intensive care unit; SAPS II - simplified acute physiology score II; RRT - renal replacement therapy; AKI - acute kidney injury; KDIGO - kidney disease improving global outcomes; LOS - length of stay
The majority of patients were classified as KDIGO stage 3 (51.9%), 25.8% KDIGO stage 2 and 22.3% as KDIGO stage 1. During ICU admission, 77.4% patients required mechanical ventilation, 72.7% required vasopressors, 27.1% required renal replacement therapy (RRT) and required a mean fluid balance within the first 48 hours of 4.6±5.4 litres. Thirty percent of patients were exposed to nephrotoxin’s. These patients had a length of stay in ICU of 10.0±10.0 days and in hospital of 36.7±38.6 days. The in-hospital mortality in this cohort of septic-AKI patients was 35.8% (KDIGO stage 1 - 13.3%, KDIGO stage 2 - 16.8%, KDIGO stage 3 - 69.9%, p<0.001).
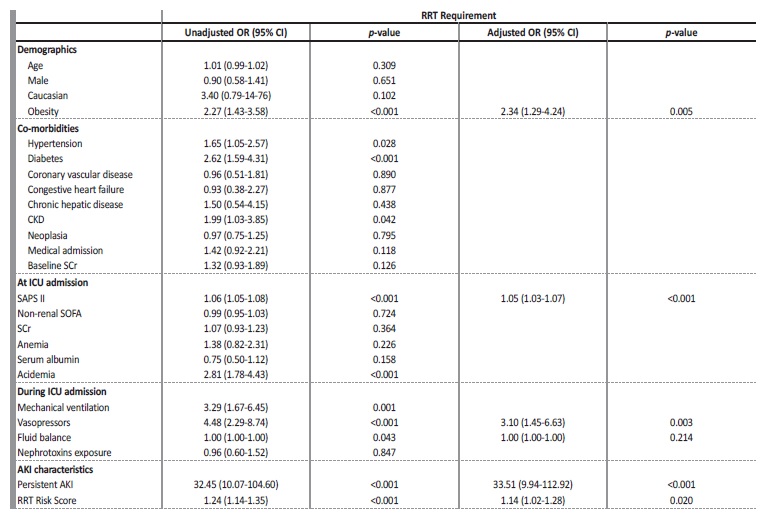
Septic-AKI patient who required RRT had a mean age of 65.4±14.6 years, mainly caucasian (98.1%), males (55.6%) and the main diagnosis at ICU admission was medical in nature (62.0%). There was no significantly difference in the mean age, race or gender between those who needed RRT and those who did not. There were also no significant differences on baseline creatinine (1.3±0.7 vs 1.2±0.5 mg/dL, p=0.120) or admission creatinine (2.6±1.6 vs 2.4±1.5 mg/dL, p=0.365). Septic-AKI patient who required RRT was more likely to be obese (40.7% vs 24.7%, p=0.002), reaching statistical significance at the multivariable analysis (adjusted OR 2.34 (1.29-4.24), p=0.005) (Table 4).
Table 4 Univariate and multivariate analysis of factors predictive of RRT requirement in Septic-AKI patients
CKD - chronic kidney disease, SCr - serum creatinine; ICU - intensive care unit; SAPS II - simplified acute physiology score II; RRT - renal replacement therapy
Septic-AKI patients who required RRT within the hospital admission had a higher incidence of hypertension (54.6% vs 42.2%, p=0.028) and diabetes (32.4% vs 17.2%, p<0.001). At ICU admission, higher SAPS II (62.3±16.6 vs 46.0±15.5, p<0.001) and presence of academia (pH<7.35) (53.7% vs 29.2%, p<0.001) were associated with the requirement of RRT (Table 3).
Need for mechanical ventilation (89.8% vs 72.9%, p<0.001) and vasopressors (89.8% vs 66.3%, p<0.001) were also associated with the need of RRT (Table 3).
Renal Replacement Therapy Risk Score
The Risk Score correlated with the requirement of RRT in septic-AKI patients (6.6±2.5 vs 5.1±2.6, p<0.001) (Table 4).
An adjusted multivariate analysis to demographic, clinical, ICU admission variables and during ICU stay factors was conducted, in which a higher RRT Risk Score remained as an independent predictor of increased risk of requirement of RRT in septic-AKI patients (6.6±2.5 vs 5.1±2.6, p<0.001; unadjusted OR 1.24 (95% CI 1.14-1.35), p<0.001; adjusted OR 1.14 (95% CI 1.02-1.28), p=0.020) (Table 4).
To assess the discriminative ability of this risk score for predicting RRT requirement, a ROC curve was produced. The AUC for requirement of RRT prediction in septic-AKI was of 0.658 (p<0.001) (Fig. 2).
The optimal cut-off was assessed to be >6, which has a sensitivity of 63.0% and specificity of 39.1%.
DISCUSSION
In this retrospective cohort, we demonstrated that a higher RRT
Risk Score calculated at ICU admission was independently associated with the need of RRT in septic-AKI patients (Table 3).
AKI is a major complication in ICU patients and is associated with worse outcomes, including prolonged in-hospital stay and increased mortality.5-7 Several urine and serum biomarkers of kidney injury have been identified over the past years for early detection of AKI, but they are not used in routine clinical care.26-28 The most commonly used
clinical (UO) and serum biomarkers (SCr and cystatin C) are unspecific and fail on the early detection of AKI. Moreover, these biomarkers fail to identify which patients will require RRT.29-32
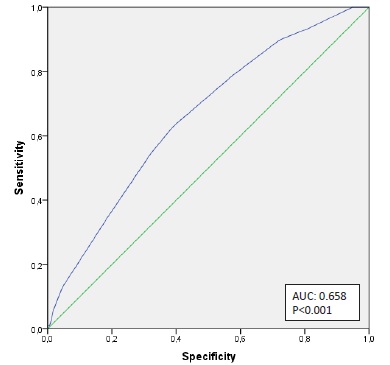
Figure 2 AUC of the risk model for the prediction of Renal Replacement Therapy requirement in septic-AKI patients.
There is no consensus about the optimal timing for the initiation of RRT. There is a controversy between those who advocate an early strategy for the initiation of RRT, without waiting for the development of AKI complication, and those more conservative who are more prone to a late strategy for the initiation of RRT, waiting for the development of acidemia, hyperkaliemia, fluid overload or uremic syndrome, thus allowing for a potential renal recovery and avoiding complications associated with RRT.
Recent randomized studies failed to determine the optimal timing for the initiation of RRT, increasing the uncertainty about the optimal strategy.33-35 The ELAIN trial is the only study to demonstrate a better survival in patients who started RRT earlier. In this study, patients required plasma neutrophil gelatinase-associated lipocalin level (NGAL) higher than 150 ng/mL to be included and only 9% of patients in the delayed strategy group did not require RRT start.36 Interestingly, in most recent studies around 40%-50% of patients in the delayed strategy group did not require RRT start.10,37,38 This highlights the need to determine which patients will, in fact, require RRT.
A recent study of Malhotra et al9 prospectively analyzed 1300 ICU patients and successively developed a risk score model that can identify patients at high risk to develop AKI, by integrating chronic comorbidities and acute events at ICU admission. In the multivariate model, chronic kidney disease, chronic hepatic disease, heart failure, hypertension, coronary vascular disease, nephrotoxin exposure, acidemia, mechanical ventilation and anemia were identified as independent predictors of AKI. The risk model developed based on these variables established an optimal cutoff value of ≥ 5 points with 31.8% of positive predictive value and 95.4% for negative predictive value.
The aim of our study was to adapt this score and try to apply it as a predictor for RRT requirement on a population of septic-AKI patients.
Our retrospective analysis studied the chronic comorbidities and acute events at ICU admission that were used in the score proposed for Malhotra et al9 with the believe that it could also be used as tool for predicting the need of RRT.
This risk stratification could allow an early identification of patients who are more likely to need RRT and implement therapeutic strategies without waiting for severe uremic complications to develop or avoid unnecessary invasive procedures in fragile patients who might recover renal function and not require RRT.
We validated this risk score as predictor of the need for RRT in septic-AKI patients. The optimal cutoff value for the prediction of needing RRT has ≥ 6 points (Fig. 2). With this score value we obtain a 63.0% sensitivity and 39.1% specificity, which means that if patient’s score is < 6, he is only 37% chance of requiring RRT.
The strong negative predictive value of this score could be used to justify a more conservative approach. A low score reflects a probability of requiring RRT of less than 40%, meaning we could delay RRT initiation and avoid this invasive procedure, which carries several risks including: mechanical complications associated to dialysis catheter insertion, increased infectious risk and hemodynamic and biochemical changes related to the technique which may delay renal recovery and contribute to myocardial ischemia.
Several studies reported chronic kidney disease, chronic hepatic disease, heart failure, hypertension, coronary vascular disease, nephrotoxin exposure, acidemia, mechanical ventilation and anemia as major risk factors for AKI,39-42 but few addressed the risk factors for the requirement of RRT once AKI is established.
Some reports already validated several biomarkers as predictors of the need for RRT, like Inhibitor of metalloproteinase-2 (TIMP-2), insulinlike growth factor-binding protein 7 (IGFBP7), urinary neutrophil gelatinase-associated lipocalin (uNGAL) or fibroblast growth factor 23 (FGF23).26-28 Cho et al26 prospectively studied a population of 124 patients diagnosed with AKI and demonstrated that TIMP-2 and IGFBP7 were independent predictors of renal replacement therapy at the time of AKI diagnosis (OR 5.75 and 44.98, respectively). Albeladi et al27 prospectively analyzed a population of 75 patients, and among those 21 developed AKI and 17 required RRT. Maximum urine levels of uNGAL measured over the first and second 24 hours of an ICU admission were highly accurate predictors of the future need for RRT (p<0.001). Fayed et al28 studied 30 patients admitted to an ICU with acute kidney injury and showed that FGF23 levels were significantly higher in patients whoneeded RRT than in other participants (mean level: 529.5 vs 285.11 pg/mL, p=0.04). Thiengo et al43 analyzed 120 patients and found that Troponin I at admission in the ICU strongly correlated with the need of dialysis in septic shock (TnI > 0.42 ng/mL - HR 3.48 [95% CI 1.69-7.18]).
Nevertheless, these studies are mainly based on biochemical markers which are not routinely used, mainly for their high associated costs, and had small number of patients. This is the first study based on clinical variables that could successfully predict the requirement for RRT in septic-AKI patients.
Several limitations of our study must be addressed. Firstly, this was a retrospective single center analysis, which diminishes the power of the study. Another important limitation to this score, is the exclusion of criteria such as the presence of diabetes and use of vasopressors during UCI stay, which have a strong correlation with the need for RRT, but further investigation is needed to confirm this association.
Finally, due to the selection of this population, these conclusions can only be applied to septic-AKI patients. Further investigation is needed to study the application of this score to other causes of AKI.
Our study has some important virtues. This is the first study to assess a valid clinical score that successfully predicts which septic-AKI patients are more likely to require RRT. This is a simple score, easily calculated at admission, derived from patients’ demographics, chronic comorbidities and acute risk factors easily obtained in routine clinical practice.
The study had a significant number of participants in a selected population, empowering the conclusions in this group of septic-AKI patients.
CONCLUSION
We developed a new easily calculated risk score to predict RRT requirement in septic-AKI patients, which combines patients’ demographics, chronic comorbidities and acute risk factors. This can be a valuable tool in clinical practice, helping clinicals on the decision whether to initiate or not RRT when the patient does not fulfill the classic indications (severe acidemia, hyperkalemia, fluid overload or uremic syndrome).