INTRODUCTION
Vasculitis associated with ANCA antibodies is a systemic autoimmune disease that courses with small blood vessels inflammation. It may sometimes be limited to the kidney, an entity known as renal limited vasculitis.1-3 The glomerular inflammatory damage may lead to capillary basement membrane rupture, which in turn may trigger fibrinoid necrosis and crescents formation, disrupting the glomerular architecture.
This translates clinically into a rapidly progressive kidney injury.4,5 For this reason, renal replacement therapy (RRT) is frequently required at diagnosis.6 Immunosuppressive (IS) agents used in induction and maintenance therapies, although essential for treatment, are responsible for a large part of the morbidity and mortality.7,8 Accordingly, an effort has been made in order to decrease immunosuppression burden in these patients, by reducing cumulative dosages of cyclophosphamide and corticosteroids.9,10 In spite of the strong induction IS treatment advocated, around 35% remain dialysis dependent 4 months after diagnosis.11 This highlights the importance of defining risk factors associated with a worse renal prognosis in order to select patients who would benefit the most from IS therapy when it comes to renal limited vasculitis. Kidney biopsy may have an important role in this patient selection.12,13 Taking this into consideration, we proposed to retrospectively evaluate a ten-year group of patients diagnosed, at our department, with renal disease induced by ANCA associated vasculitis (AAV).
METHODS
This was a retrospective observational study with the aim of characterizing the patient cohort with the inaugural diagnosis of AAV, treated in our Nephrology department.
The aims of our evaluation were:
- Evaluate patient demographics and clinical presentation;
- Evaluate kidney outcomes on discharge and at 6 and 12 months;
- Evaluate patient survival on discharge and at 6 and 12 months;
- Evaluate treatment outcomes during the follow-up period.
Data were collected from clinical records of all patients admitted to the Coimbra’s University Hospital Nephrology Department with the diagnosis of ANCA vasculitis, between 1st June 2009 and 1st June 2019.
We selected for further analysis all patients with the inaugural diagnosis of AAV that had at least one year of follow-up. Data were analyzed with SPSS v26®, using descriptive analysis, parametric and non-parametric tests, ROC curves and survival analysis.
Results were considered statistically significant if the p-value was inferior to 0.05. Prognosis was measured as the percentage of patients on hemodialysis, as well as mortality rate, at 6 and 12 months after diagnosis.
Biopsies were scored as 1 (focal class), 2 (crescentic), 3 (mixed) and 4 (sclerotic), according to glomerular involvement by the AAV. Interstitial fibrosis and tubular atrophy were also graded (1: <10%; 2: 10%-25%; 3: 25%-50%; 4: >50%).
Induction therapy with either cyclophosphamide or rituximab was given to patients with organ or life-threatening disease. Cyclophosphamide was either given orally (2 mg/kg/day with adjustments according to frailty and kidney function) or intravenously (according to CYCLOPSEUVAS protocol, with adjustments to age and kidney function); rituximab was administered intravenously (375 mg/m2 per week for four weeks or two doses of 1 g with an interval of 14 days) - all patients that received rituximab also performed IV cyclophosphamide (usually 2-3 cycles). Corticosteroid intravenous (IV) pulses were usually provided (from one to three pulses of 250 to 1000 mg of methylprednisolone), followed by oral prednisolone therapy with progressive dose tapering.
Patients received plasmapheresis if they had severe kidney disease, pulmonary hemorrhage or overlap between ANCA and anti-GBM disease. All patients treated with IS received pneumocystis jirovecii prophylaxis with trimethoprim-sulfamethoxazole and all patients treated with cyclophosphamide received oral candida infection prophylaxis with nystatin; all patients performing IV cyclophosphamide also received hemorrhagic cystitis prophylaxis with mesna.
RESULTS
1. Patient Population
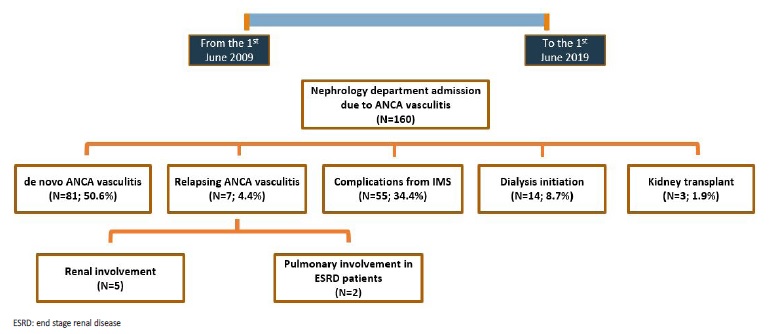
During the study period there were a total of 160 admissions in our Nephrology Department with the diagnosis of AAV, comprising a total of 88 patients. Among these, there were 81 (50.6%) cases of de novo vasculitis, 7 (4.4%) relapses, 33 (20.6%) admissions due to complications from induction IS therapy, 22 (13.8%) due to complications from maintenance IS therapy, 14 (8.8%) admissions in order to initiate dialysis and 3 (1.9%) hospitalizations for renal transplant (Fig.1). Most AAV relapses were associated with kidney injury (N=5; 71.4%), while two relapses presented with diffuse alveolar hemorrhage in end stage renal disease (ESRD) patients.
Inaugural cases were mainly due to anti-MPO antibody associated vasculitis (N=69; 85.2%). Double antibody positivity was found in 9 cases (11.1%), always with the same combination: anti-MPO plus anti-GBM. Mean age at diagnosis was 67.4 ± 15.7 years old and most patients were male (N=47; 58%). Unless mentioned otherwise, whenever we refer to AAV in the results section of this paper we are referring to de novo AAV cases.
2. Initial presentation
Fig. 2 briefly resumes AAV clinical presentation across the three groups (anti-PR3, anti-MPO and anti-MPO + anti-GBM), as well as kidney outcomes at discharge. All the inaugural cases presented with kidney injury. The majority of patients (N=41; 50.6%) presented with acute on chronic kidney disease. At admission, patients had a mean serum creatinine (sCr) of 6.8 ± 4.0 mg/dL. Dialysis was required in 23 (28.4%) patients at admission and 45 (55.6%) at any time during hospitalization, while 36 (44.4%) of patients depended on RRT at discharge. The worst renal outcome was observed in patients with both, anti-MPO and anti-GBM antibodies, bearing the highest mean sCr at admission (10.8 ± 3.0 mg/dL; p=0.002), as well as the greatest percentage of patients on dialysis at admission (66.7%; p=0.019) and at discharge (100%; p=0.002). Anti-PR3 associated vasculitis had a more aggressive presentation than anti-MPO, with a higher Birmingham Vasculitis Activity Score (BVAS) (22.7 ± 6.1 vs 14.8 ± 4.2), higher sCr (6.9 ± 3.5 mg/dL vs 6.2 ± 3.9 mg/dL) and more patients requiring hemodialysis at diagnosis (33.3% vs 21.7%), even though only BVAS differences reached statistical significance.
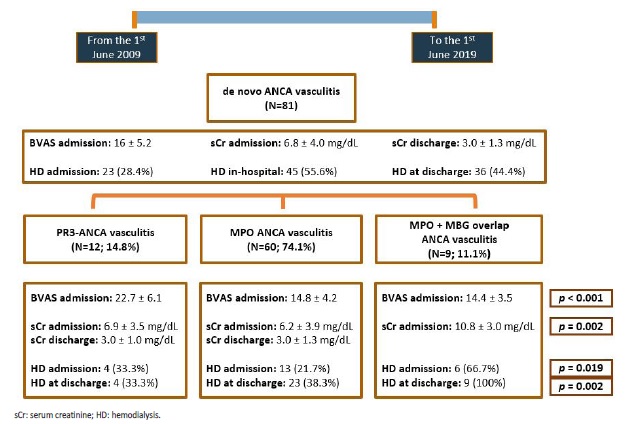
Figure 2 Flowchart representation of the main clinical and analytical variables among patients with an initial diagnosis of ANCA vasculitis. p-values are provided only for differences with statistical significance (p<0,05). Mean serum creatinine at discharge was obtained from nondialysis dependent patients.
The mean BVAS at admission was 16 ± 5.2. The most frequent extra-renal symptoms/signs were constitutional (50.6%) and respiratory (22.2%), as shown in Table 1. Renal limited vasculitis was diagnosed in 29 (35.8%) patients.
As we can see in Table 2, only sCr at admission differs significantly across AAV subtypes. The remaining analytical variables present relatively overlapping results in the selected groups.
Table 1 BVAS at admission in patients with de novo ANCA associated vasculitis. The items in bold correspond to the most frequent organ system involvement (besides the kidney), as well as the mean BVAS.
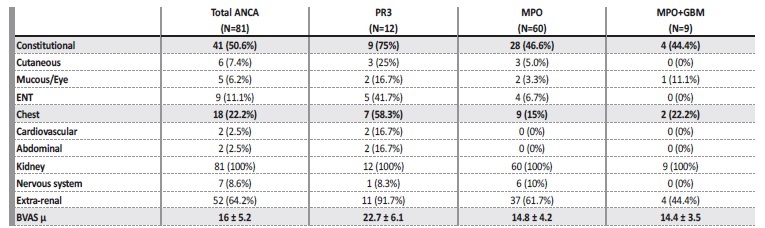
ENT: ear, nose and throat.
Table 2 Analytical results, at admission, in patients with de novo ANCA associated vasculitis.
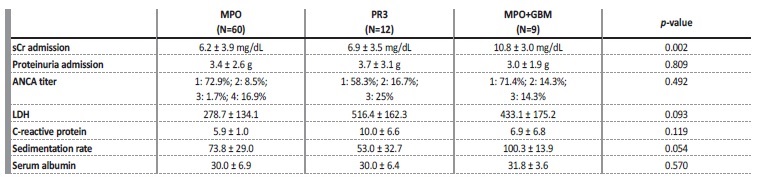
sCr: serum creatinine. LDH: lactate dehydrogenase. ANCA titer - 1: 0-150 UI/mL; 2: 150-300 UI/mL; 3: 300-600 UI/mL; 4: >600 UI/mL
a. Kidney biopsy analysis
Biopsy results were available in 38 (46.9%) patients. Biopsy samples were inadequate (insufficient number of glomeruli) in 9 (11.1%) patients. Information about interstitial fibrosis/tubular atrophy (IFTA) was not precise in 11 (13.6%) patients. Biopsy results, according to recently proposed classification (1: focal, 2: crescentic, 3: mixed and 4: sclerotic), as well as IFTA grade, are presented in Fig. 3.
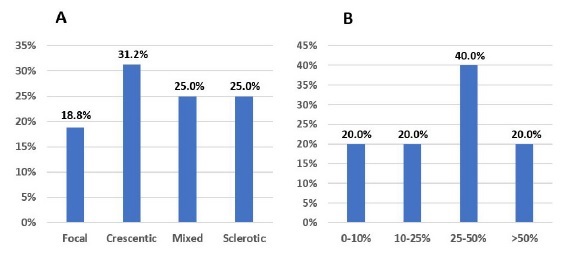
Figure 3 A: Biopsy results, according to glomerular histopathologic score, in patients with de novo ANCA associated vasculitis. B: Biopsy results, according to IFTA grade, in patients with de novo ANCA associated vasculitis.
Higher IFTA grade (>25%) was associated with mixed and sclerotic classes (vs. focal and crescentic classes): 73.3% of biopsies with IFTA>25% were classified either as a mixed or sclerotic class (p=0.004). There were no differences in the medians of proteinuria and sCr at admission, across the different glomerular lesion and IFTA scores.
b. Treatment
Induction IS agents differed along the time span of the study. Most patients (66.7%) received cyclophosphamide; however, oral cyclophosphamide was mainly used up until 2016 - since then, all patients using cyclophosphamide used the CYCLOPS-EUVAS IV protocol; rituximab was mostly used since 2016. Decision not to treat (all cases in patients with renal limited vasculitis that were dialysis dependent on admission) was made in 17 (21%) patients (Fig. 4).
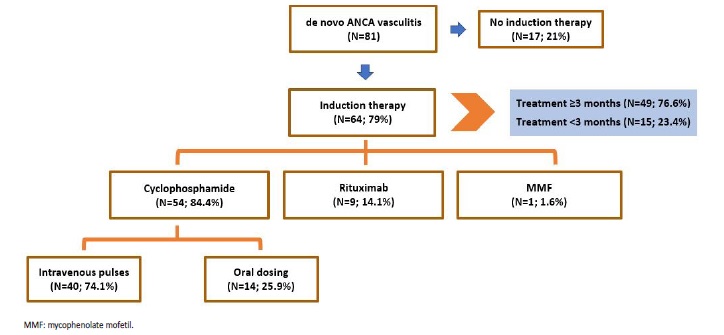
Figure 4 Schematic representation of treatment decisions in patients with de novo ANCA associated vasculitis.
Among treated patients, it was not possible to achieve the minimum of 3 months of treatment in 15 (23.4%) cases, 7 (46.7%) due to immunosuppression associated infections and 8 (53.3%) due to patient death. Most deaths (75%) were also related to infections, presumably associated with IS therapy.
Intravenous corticosteroids were administrated to most patients (N=74; 91.4%), from one to three pulses of 250 to 1000 mg. Plasmapheresis was provided to 30 (37%) patients: in 11 patients
(36.7%) due to severe kidney disease (sCr>5 mg/dL and dialysis requirement), in 10 cases (33.3%) of diffuse alveolar hemorrhage and 8 (26.7%) patients (30%) with overlap between anti-MPO and anti-GBM; one patient received plasmapheresis because he had a severe clinical presentation, consisting of sudden blindness and acute kidney failure (not requiring dialysis) with high anti-MPO titers (>600 UI/mL).
c. Kidney and patient survival
In relation to renal outcomes, most patients (61.4%) were dialysis dependent at 12 months post diagnosis. Mean sCr at 6 and 12 months of follow-up, from non-dialysis-dependent patients is provided in Fig. 5.
Across the three groups of AAV, the only statistically significant differences in outcome were dialysis dependency at discharge (Fig. 2), and at 6 and 12 months after diagnosis (Fig. 5), mainly due to the worse prognosis in patients with overlap ANCA/GBM disease, as there was no difference between anti-MPO and anti-PR3 AAV. Median hospitalization time at first admission (at the time of diagnosis) was 21 days (minimum: 2 days; maximum: 145 days).
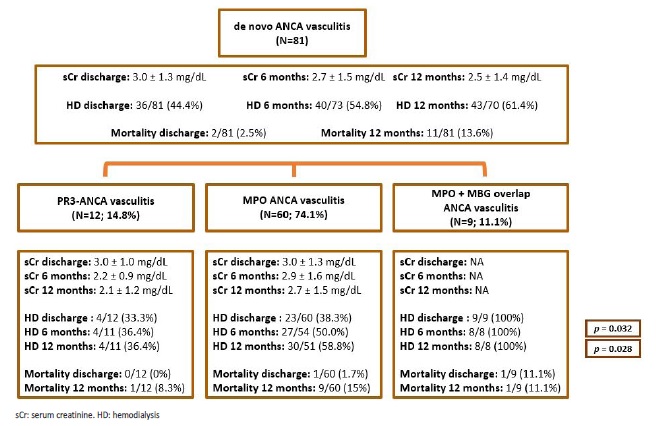
Figure 5 Diagram illustrating kidney function evolution, as well as kidney and patient survival, in patients with de novo ANCA associated vasculitis. p-values are provided only for differences with statistical significance (p<0.05). Mean serum creatinine values were obtained from non-dialysis dependent patients.
In relation to patient survival, in hospital mortality rate during initial hospitalization was 2.5% (N=2/81); it increased to 13.6% (N=11/81) at 12 months from diagnosis. Deaths were mostly (N=8; 72.7%) due to infections: five pneumonias (pseudomonas aeruginosa, klebsiella pneumoniae ESBL + aspergillus terreus, methicillin-resistant staphylococcus aureus, Influenza A, one case without microorganism identification); one acute pyelonephritis due to carbapenemase-producing klebsiella pneumoniae; one case of bacteraemia due to carbapenemase-producing klebsiella pneumoniae and one dialysis catheter-related sepsis due to methicillinresistant staphylococcus aureus.
There was no difference in patient survival at 12 months between the different sub groups of AAV and no difference in patient or kidney survival at 12 months between the different IS strategies (Fig. 6). Most notably, patients with renal limited AAV that received no induction therapy had a similar 12 months survival, in relation to the other subgroups.
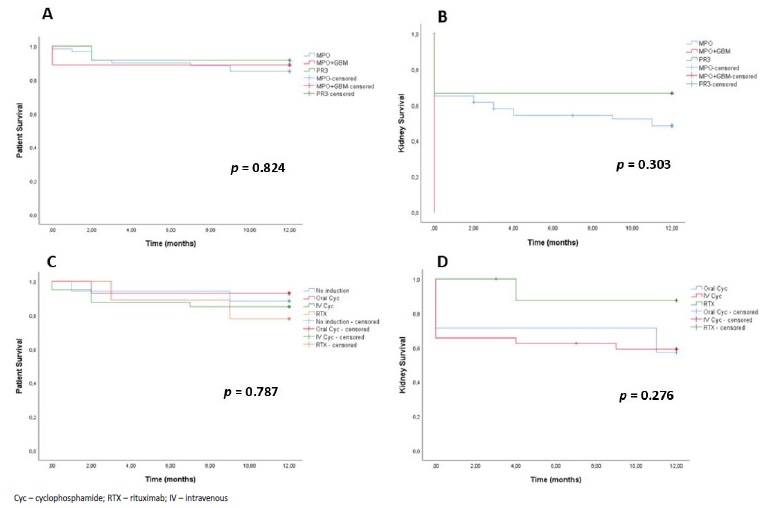
Figure 6 A: Patient survival during the first year of follow-up in patients with de novo ANCA associated vasculitis, according to ANCA type. B: Kidney survival during the first year of follow-up in patients with de novo ANCA associated vasculitis, according to ANCA type. C: Patient survival according to induction treatment strategy. D: Kidney survival according to induction treatment strategy (only patients treated for ≥3 months).
d. Kidney recovery in dialysis-dependent patients at discharge
Among dialysis dependent patients at discharge (N=36; 44.4%), treatment for at least 3 months was provided in 15 (41.7%) cases (Fig. 7). Two of these patients partially recovered their kidney function, becoming temporarily independent of dialytic treatment. Both had anti-MPO AAV and were treated with IV corticosteroid pulses, plasmapheresis and IV cyclophosphamide (cyclops protocol). Their mean sCr at 12 months was 4.5 and 5.7 mg/dL, respectively. The first patient (sCr-4.5 mg/dL) was able to stop dialysis at 4 months and started peritoneal dialysis 4 years later. The second patient (sCr-5.7 mg/dL) suspended hemodialysis after 3 months and restarted it 12 months after.
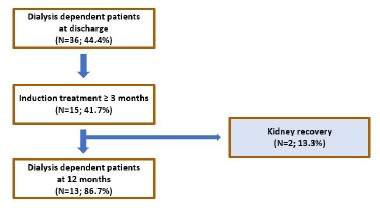
Figure 7 Flowchart illustrating the effect of induction treatment in kidney recovery among dialysis-dependent patients at discharge.
e. Prognostic factors for kidney survival
Statistical evaluation of potential prognostic factors for kidney survival are exhibited in Table 3. Only patients who received induction immunosuppression for at least 3 months were considered. Using ROC curve analysis for sCr, estimated glomerular filtration rate (eGFR) and proteinuria at admission, we were able to obtain the best cut-off point for these variables, in order to discriminate patients in terms of dialysis dependency at 12 months. However, neither of these cut-off values were sufficiently specific to be used as adequate discriminators. The best cut-off identified was sCr-8.1 mg/dL at admission (AUC-0.8; CI: 0.66-0.94), with a sCr>8.1 mg/dL having a sensitivity of 76.5% and a false positive ratio of 18.8% for identifying patients that will be dialysis dependent at 12 months.
Table 3 Evaluation of potential prognostic factors for kidney survival (dialysis dependency at 12 months). Odds ratio are only provided when statistically significant (p<0.05). Only patients who received induction immunosuppression for at least 3 months were analyzed. Cut-off values for proteinuria, serum creatinine and eGFR were obtained from ROC curve analysis
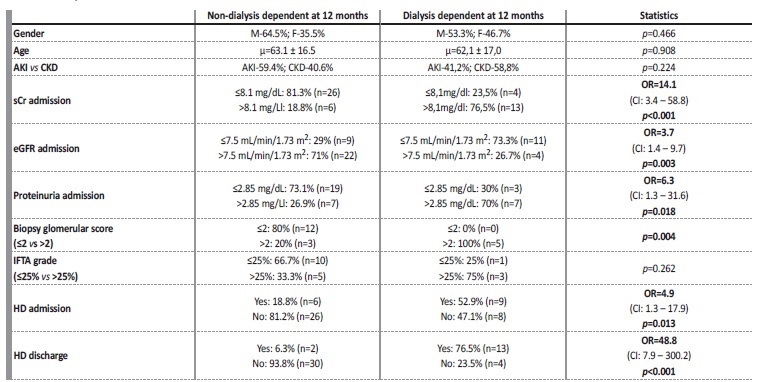
OR: odds ratio. Items in bold represent variables with statistically significant impact in renal outcome (dialysis at 12 months). CI: confidence interval. sCr: serum creatinine. eGFR: estimated glomerular filtration rate. HD: hemodialysis. IFTA: interstitial fibrosis/tubular atrophy.
Higher sCr (OR=14.1; p<0.001) and lower eGFR (OR=3.7; p=0.003) at admission, as well as dialysis requirement at diagnosis (OR=4.9; p=0.013) and its dependency at discharge (OR=48.8; p<0.001) were identified as potential prognostic markers, as they had a statistically significant impact on kidney survival (dialysis dependency at 12 months). Higher proteinuria at admission (OR=6.3; p=0.018) also predicted worse kidney outcome.
Non-treated patients and those treated for less than 3 months had a mean sCr of 6.4 ± 2.9 mg/dL, a mean eGFR of 9.5 ± 5.7 mL/min/1.73 m2 and a mean proteinuria of 3.7 ± 2.4 g, at the time of diagnosis. Fig. 8 illustrates kidney biopsy results.
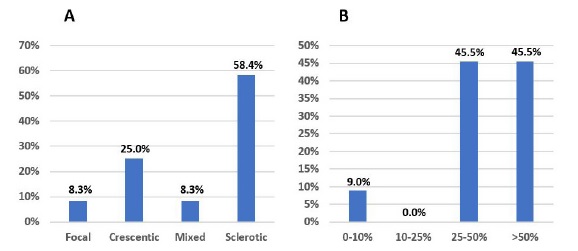
Figure 8 A: Biopsy results, according to glomerular histopathologic score, in patients treated for <3 months. B: Biopsy results, according to IFTA grade, in patients treated for <3 months.
These patients had mainly sclerotic class (58.4%) and 45.5% had high grade IFTA (>50%). Biopsy results were unavailable in 21 (65.6%) cases. Only four patients had lower grade biopsy lesions: one presented focal class; three showed a crescentic class and one of these had low grade IFTA (<25%). Three of these four patients did not reach the minimum treatment time because they died from infectious complications associated with IS therapy; immunosuppression was also withdrawn in the other patient because of associated infectious complications.
DISCUSSION
ANCA associated vasculitis epidemiology has changed in the last decades, with the peak age at diagnosis increasing from 70 years old to between 80 and 85 years old.14 Our patient cohort has a mean age more similar to previous reports. There are also reports of a slight male predominance which is present in our patient sample as well.14 Disease severity, measured by the BVAS at admission, was somewhat lower in our study sample compared to what has been published (16 vs 17.9).15 These data must be interpreted in a specific context, as our patient sample includes 29 (35.8%) patients with renal-limited, which may partially explain the lower vasculitis activity score. Concerning kidney involvement, our patient sample had a higher sCr (6.4 ± 4.0 mg/dL) than what has been published (5.6 ± 4.4 mg/dL).16 Nonetheless, our dialysis dependent patient’s rate at diagnosis (28%) was similar to that of some reports.17 Positivity for anti-GBM was associated with higher sCr at admission, as well as dialysis necessity at diagnosis and its dependency at discharge.
This clinical presentation and kidney outcome is more similar to anti-GBM associated glomerulonephritis, in agreement with previous reports.18,19
Biopsy results revealed more chronic glomerular lesions in our patient sample than what has been found in previous reports, with sclerotic class representing 25% of biopsy samples (vs 13% and 16.7%) and focal class accounting only for 18.8% (vs 26% and 28.3%).12,20
However, we should add that adequate biopsy information was available only in 38 (46.9%) patients, which may have caused some bias.
Treatment of ANCA associated vasculitis has evolved throughout the last decades, from high dose oral alkylating agents (cyclophosphamide) to progressively lower dosing schemes of IS agents, such as the cyclops (cyclophosphamide) and the pexivas (systemic corticosteroids) protocols, with the intent of reducing toxicity from IS therapy.7,8 This effort to reduce cumulative dose of immunosuppressors reflects the general concern about the infectious complications associated with these therapies. In fact, infections are the main cause of death among these patients, particularly during the first year following AAV diagnosis.21-23In our study sample, most (72.7%) deaths were due to infections, presumably related to immunosuppression.
ANCA associated vasculitis is, in many patients, a systemic disease, and patients with extra-renal manifestations need IS treatment to remit the disease and avoid chronic organ damage. However, in patients with renal limited AAV, we believe it is important to select those who might benefit from induction treatment and distinguish them from patients who probably will not recover kidney function and might be unnecessarily exposed to a higher infectious risk. This rationale has already been put into practice in renal limited anti-GBM disease; in this pathology, current KDIGO guidelines recommend abstaining from IS in patients without alveolar hemorrhage that are oliguric, with advanced dialysis-dependent kidney failure, especially if they have a very high proportion of crescents in their kidney biopsy.24However, in renal limited AAV, current guidelines recommend immunosuppression discontinuation only after 3 months in patients with renal limited vasculitis who remain dialysis-dependent.24 We believe that, in patients with renal limited AAV, it is critical to select patients who might benefit from induction IS treatment, eventually applying the same reasoning that is already standard in anti-GBM disease.
In order to avoid bias resulting from decision not to treat patients with apparently smaller renal recovery chance, we only looked for prognostic markers in patients provided with at least 3 months of induction IS treatment. In our patient sample, dialysis necessity at diagnosis (OR=4.9; p=0.013) and, particularly, its dependency at discharge (OR=48.8; p<0.001) were highly associated with dialysis dependency at 12 months post-diagnosis. In fact, only two (13.3%) patients who were dialysis dependent at discharge recovered their kidney function. Both patients became dialysis-free less than 6 months after diagnosis. Although these patients partially recovered, allowing them to be temporarily without RRT, they remained with a severely impaired kidney function (sCr of 4.5 mg/dL and 5.7 mg/dL at 12 months, respectively).
Besides, the patient with worse renal function returned to dialysis after only 12 months, which makes us wonder whether it was worth to take the infectious risk associated with induction therapy.
Other than dialysis dependency at diagnosis and discharge from the first hospitalization, we found that, at the time of diagnosis, the presence of sCr >8.1 mg/dL (OR=14.1; p<0.001), eGFR ≤7.5 mL/min/1.73 m2 (OR=3.7; p=0.003) and proteinuria >2.85 g (OR=6.3; p=0.018) were indicators of dialysis dependency at 12 months. A higher amount of proteinuria at diagnosis may be more common in patients who present later in their disease course, with more chronic lesions in their kidney biopsy. However, we did not find such a relation in our sample, which could be related to the lack of biopsy results, as mentioned earlier. The associations found between sCr and eGFR with worse kidney outcome are relatively more straightforward as these patient’s kidney function is already severely impaired at diagnosis. Although an association between worse kidney survival and higher IFTA grade and/or more chronic glomerular lesions has already been reported, we were not able to confirm these results in our study sample.12,13,20
Even though we found a statistically significant association between the biopsy glomerular score (≤2 vs. >2) and dialysis dependency at 12 months, we were not able to obtain an odds ratio because we lacked kidney biopsy information in dialysis-dependent patients at 12 months who were treated for at least 3 months (see Table 3). Moreover, unfortunately, kidney biopsy was not performed at diagnosis in the two patients with kidney recovery, so we cannot clarify if they had more acute/recent parenchymal lesions. Furthermore, we only analyzed patients treated for a minimum of 3 months, in order to avoid bias related to non-treatment. However, by avoiding “untreated” patients, we may also be biasing our analysis and limiting the identification of histopathological risk factors, as most of the non-treated patients presented chronic lesions on renal biopsy (58.8% with sclerotic class; 45.5% with IFTA>50%).
We believe that IS therapy withdrawal at the time of discharge should be considered in patients with renal limited AAV who remain dialysis-dependent at discharge, avoiding the exposure to the risks of 3 months of unnecessary IS, as is already done in anti-GBM disease.
We consider our opinion is supported by the fact that the majority of deaths in our sample were due to infections associated with IS therapy, and considering that most dialysis-dependent patients at the time of discharge remain so after 12 months. Finally, we think that this decision should, if possible, be supported by kidney biopsy features, considering the suspension of immunosuppressants in patients with sclerotic class and/or high-grade IFTA (>50%).