INTRODUCTION
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis are systemic autoimmune diseases that affect small-sized blood vessels and are accompanied by the presence of ANCAs in the serum.
This disease entity can be classified either according to ANCA specificity, differentiating between anti-proteinase 3 (PR3-ANCA) and antimyeloperoxidase (MPO-ANCA) ANCA-associated vasculitis, or by their phenotype, distinguishing between granulomatosis with polyangiitis, microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis and renal-limited vasculitis.1
Renal involvement is a common and severe feature of ANCAassociated vasculitis, usually characterized by rapidly progressive glomerulonephritis with kidney failure, hematuria and glomerular proteinuria, leading to end-stage kidney disease (ESKD) and death in a considerable number of patients. In untreated patients, there is a reported mortality of 80%-90%. Following the introduction of immunosuppressant therapies, the 5-year survival has been steadily rising to around 70%-80% and data also suggest that there are ongoing improvements in ESKD.2
Treatment for ANCA-associated vasculitis includes potentially toxic immunosuppressive agents. Although immunosuppressive regimens have significantly improved mortality, treatment complications remain a major problem. Treatment related toxicity, such as infection, has been reported as being responsible for the majority of deaths in the first year following diagnosis, as well as conferring a long-term risk of malignancy.3 Immunosuppression is currently not tailored to biopsy findings, in contrast to lupus nephritis, for which treatment is adjusted according to histological classification.
One of the challenges of ANCA-associated glomerulonephritis is to ascertain histological features of prognostic value, including identification of pathological features in patients who may benefit of immunosuppression and to identify patients who will progress to ESKD in whom heavy immunosuppression therapy is likely to do more harm than good.
RENAL PATHOLOGY IN ANCA-ASSOCIATED VASCULITIS
Although rapid progressive glomerulonephritis and a positive ANCA test are diagnostic of ANCA-associated vasculitis, histologic confirmation from a diagnostic kidney biopsy is still considered the gold standard to diagnose ANCA-associated vasculitis. Renal involvement of ANCAassociated vasculitis is characterized by the so called pauci-immune glomerulonephritis, which often presents as necrotizing or crescentic glomerulonephritis in the absence or paucity of immune deposits on immunofluorescence.
On light microscopy, the severity of findings generally parallels the severity of clinical presentation, ranging from mild focal and segmental proliferative glomerulonephritis in patients with asymptomatic hematuria and normal or near-normal kidney function to a diffuse necrotizing and crescentic glomerulonephritis in patients with acute kidney injury. Hence, histologic analysis of biopsy can contribute not only to the diagnosis but also to predict kidney outcomes.4
Several studies found associations between histopathologic parameters in diagnostic kidney biopsies and kidney outcomes.
The most consistent findings were associations between a high percentage of normal glomeruli and a favorable kidney outcome and the presence of a high percentage of sclerotic glomeruli and a worse kidney outcome. Furthermore, the presence of active lesions such as cellular crescents correlates with a higher probability of kidney function recovery with immunosuppressive therapy.5,6
In addition to glomerular changes, tubulointerstitial findings have also been found to be associated with renal prognosis, and tubular atrophy is an especially important risk factor for impaired renal function during follow-up.7 The relationship of vascular lesions to renal outcome has been reported less frequently, although arteriosclerosis in the initial biopsy is identified as a risk factor for long-term dialysis.4
THE HISTOPATHOLOGICAL CLASSIFICATION OF ANCA-ASSOCIATED VASCULITIS PROPOSED BY BERDEN ET AL.
Taking into account the results from previous studies and with the purpose of summarizing the most important data from the renal biopsy, in order to give an indication about long-term renal outcome, a histopathologic classification of glomerulonephritis in ANCA-associated vasculitis was proposed by Berden et al in 2010.8
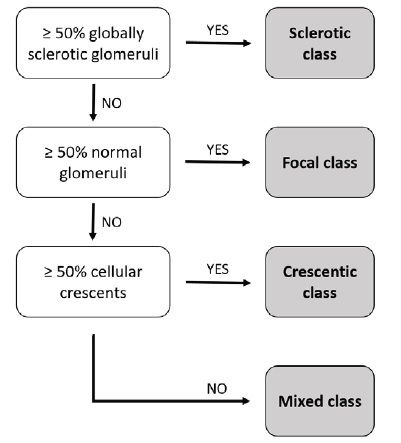
This classification is based on glomerular morphology on light microscopy and consists of four categories: focal, crescentic, mixed and sclerotic classes. The focal class is defined by the predominance of normal glomeruli (≥ 50%), the crescentic class by the predominance of cellular crescentic glomeruli (≥ 50%), and the sclerotic class by the predominance of globally sclerotic glomeruli (≥ 50%). The mixed class represents a heterogeneous glomerular phenotype in which no glomerular feature predominates (Fig. 1).
For classification purposes, all biopsies must be characterized as pauci-immune and a minimum of 10 whole glomeruli is considered adequate. Hematoxylin-eosin, methenamine silver, periodic acid-Schiff and Masson Trichome stainings are required. Furthermore, this classification does not take into account patients with comorbid diseases or overlap syndromes, such as ANCA-associated glomerulonephritis in combination with anti-glomerular basement membrane (GBM) glomerulonephritis.
This classification was subjected to a first validation study incorporated in the publication by Berden et al8 that included 100 biopsies from 32 centers across nine European countries, from patients who participated in the CYCAZAREM and MEPEX studies conducted by EUVAS. The authors demonstrated that renal biopsy categories correlated to the degree of renal function at presentation and at 1-year and 5-year follow-up. Patients in the focal category had the best renal prognosis, followed by the crescentic, mixed and sclerotic categories in that order. Patients with focal ANCA-associated glomerulonephritis present with relatively preserved renal function and have a relatively favorable renal outcome (renal survival was 93% at 1-year and 93% at 5-year follow-up).
Patients with crescentic ANCA-associated glomerulonephritis present with highly active renal disease and severely reduced renal function but stand a good chance for renal function recovery (renal survival was 84% at 1-year and 76% at 5-year follow-up). Patients with a mixed phenotype have an intermediate outcome profile (renal survival of 69% at 1-year and 61% at 5-year follow-up).
Patients with sclerotic ANCA-associated glomerulonephritis at the time of biopsy run the highest risk for not recovering renal function (renal survival of 50% at 1-year, 50% at 5-year and only 25% at 7-year follow-up) and also have a higher risk for death within the first year after diagnosis.
The Berden classification in based purely on the presence of glomerular lesions. Tubulointerstitial lesions such as fibrosis or tubular atrophy were not included in the classification system because, although they were associated with long-term renal outcome, their addition to the classification did not significantly improve its predictive value and only increased its complexity. In addition, the histopathological classification and the baseline eGFR (estimated glomerular filtration rate) were the only independent predictors for eGFR at both 1 and 5-year follow-up in a multivariable analysis that took into account patient age, treatment, baseline eGFR and the histopathological classification of ANCA-associated glomerulonephritis.
VALIDATION OF THE HISTOPATHOLOGICAL CLASSIFICATION OF ANCA-ASSOCIATED VASCULITIS
Since 2010, numerous cohorts have validated the Berden classification system, these studies are summarized in Table 1. All studies agreed that a favorable renal outcome was associated with the focal class, whereas a poor outcome was more likely in the sclerotic class, confirming that the classification system is of predictive value for renal outcome. Regarding the crescentic and mixed class however, many discrepancies exist. Several groups confirmed the initial result of Berden et al and found that patients in the crescentic category have a better outcome, while others found better renal survival in the mixed category, without always achieving statistical significance in either case. Meta-analysis have also found no significant prognostic difference between crescentic and mixed classes, with some authors merging the two classes (Table 1). While no study led to definite conclusions, it is clear that the presence of contrasting observations underlines the need to improve the initial classification.
Table 1 Studies reporting renal survival according to the histopathologic classification6,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24
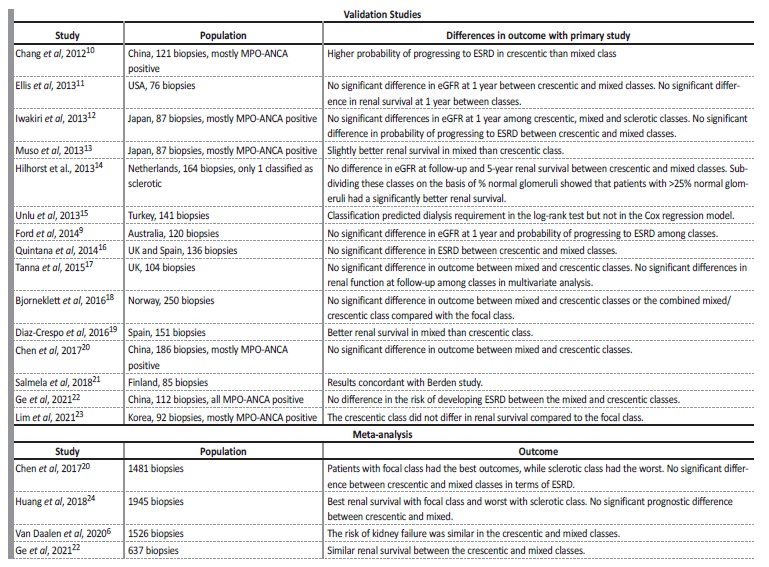
Possible explanations for the discrepancies in these results are differences in patient populations, differences in treatment and interobserver variability. Two studies tested the inter-observer reliability of the Berden classification. In a study conducted by Ford et al,9 involving 3 pathologists and 145 biopsies, they found a κ statistic of 0.46, with the worst results in the mixed class (κ =0.23). Agreement was higher for sclerotic (κ =0.70), crescentic (κ =0.51) and focal classes (κ=0.47). The authors of the original study also conducted their own reproducibility study, with fair to good agreement (κ =0.56).6 Consequently, limited reproducibility of some classes should be kept in mind.
The authors of the original study revisited this classification in 20206 and agreed that there was similar prognostic outcomes between the crescentic and the mixed classes. However, they disagreed with merging these two classes because an important histopathologic distinction would be lost. They are currently performing an analysis that evaluates histopathologic patterns in more detail, with a focus on the distinction between cellular, fibrocellular, and fibrous crescents and are evaluating the necessity to add tubulointerstitial parameters to the histopathologic classification.
THE RENAL RISK SCORE PROPOSED BY BRIX ET AL.
In the meantime, in 2018, Brix et al25 proposed a new predictive tool in the form of a risk score, based on a cohort of German patients.
Similar to the Berden histopathologic classification, its main goal is to identify those who would probably not benefit from immunosuppressive treatment because of severe renal damage from which they are not expected to recover.
It incorporates one clinical parameter and two pathologic parameters: kidney function at time of diagnosis (G1: eGFR ≤15 mL/min/1.73m2; G0: >15 mL/min/1.73 m2), percentage of normal glomeruli (N0: >25%; N1 10-25%; N2 <10%) and percentage of interstitial fibrosis and tubular atrophy (T0: ≤25%; T1 >25%). These were the variables that better correlated with renal outcome in multivariate analysis on a training cohort. Using Cox regression analysis, the points assigned to each parameter were: N1 = 4, N2 = 6, T1 = 2 and G1 = 3. Based on these parameters, a Renal Risk Score (RRS) between 0 and 11 points allows to classify patients in low (0 points), medium (2-7 points) or high (8-11 points) risk categories with respect to risk of ESKD. Using the RRS, the risk of ESKD was 0%, 16%, and 68% in low, medium, and high RRS categories, respectively, in the original cohort.
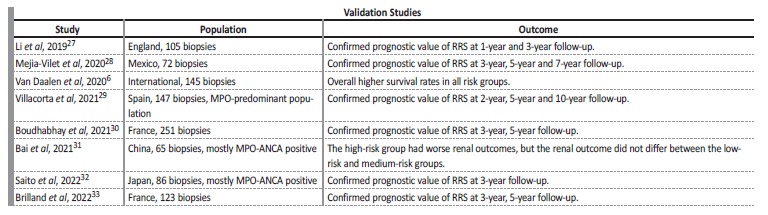
This risk score has been validated in further cohorts (Table 2). The good prognosis for patients in the low-risk group is well reproduced in the validation cohorts and a recent meta-analysis26 confirmed that the pooled incidence rate of ESRD was 4%, 22% and 58% in the low, medium and high-risk groups, respectively. There are discrepancies, however, regarding the prognosis of the high-risk group. A substantial fraction of patients classified in the worse subgroups of both ESKD classifications did not evolve towards ESKD, while some patients classified in the intermediate risk subgroups reached ESKD. Even patients with a severe disease classified as high-risk have a potential for renal improvement under treatment, therefore, cautious interpretation of the RRS in relation to treatment decisions is imperative.
FUTURE PERSPECTIVES
Kidney biopsy is more than just a diagnostic tool for ANCA-associated vasculitis, it appears to be an indispensable step in the assessment of prognosis and therapeutic decision making in these patients.
Both the Berden Histopathologic Classification and the Brix Renal Risk Score have been extensively validated in subsequent cohorts. However, although they provide valuable information to the clinician, prognostic scores and classifications do not fully predict renal and patient outcomes.
To this date, we believe there is no perfect score or tool to accurately predict the risk of ESKD at baseline, as ANCA-associated vasculitis has a multitude of different clinical scenarios. Risk stratification has the purpose of helping to manage these patients, most of them with multiple comorbidities. Personalized medicine is the holy grail. With further research, large and prospective cohorts, new clinical data (age) and serologic parameters (ANCA subtype) we will be closer to individualized treatment. Science and medicine will never stop to evolve, therefore these scores and classifications need to be constantly adapted and reviewed, as increasingly indispensable tools for treating patients.