The prevalence of chronic kidney disease (CKD) has been rising steadily around the world, but, despite numerous trials, until recently, only two classes of drugs were able to slow the deterioration of kidney function: angiotensin-converting-enzyme inhibitors (ACEi) and angiotensin- receptor blockers (ARB).1,2
A paradigm shift emerged with the introduction of sodium-glucose cotransporter 2 inhibitors (iSGLT-2), a new family of antidiabetic drugs, into clinical practice. Trials have demonstrated an attenuation of proteinuria and decline of kidney function in patients with CKD, regardless of the diabetes status.3 Kidney transplant recipients (KTR), even those without prior diabetes, are at increased risk of post-transplant diabetes mellitus (PTDM), which is associated with worse graft and vital prognosis.4 The evidence regarding the impact of iSGLT-2 in KTR is still limited, as there are no large-scale results reported to date.
We conducted a retrospective single-centre analysis of 15 KTR in whom iSGLT-2 where prescribed. The majority were female (53.3%), 86.7% were white, with a mean age of 65.1±8.6 years, kidney transplant vintage of 11.1±8.5 years, with a follow-up period of 286±134 days after the initiation of iSGLT-2. Dapagliflozin was prescribed in 86.7% of the patients. The immunosuppression regimen was tacrolimus + mycophenolate mofetil (MMF) + prednisolone in the majority of patients (66.7%), but we also analysed patients taking tacrolimus + everolimus + prednisolone (13.3%), cyclosporine + MMF + prednisolone (13.3%) and everolimus + MMF + prednisolone (6.7%). The most frequent causes of native kidney disease were autosomal dominant polycystic kidney disease (26.7%), undetermined (26.7%) and diabetic kidney disease (20%). All patients had PTDM or prior DM, with 26.7% requiring insulin. The indication for prescription of iSGLT-2 was glycemic control in 86.7% and albuminuria>500 mg/g despite maximal tolerated dose of ACEi/ARB in the remaining. Patients submitted to kidney transplant or treatment for rejection in the previous 12 months and those with history of recurrent urinary tract infections (UTI) were excluded.
In our sample, we found no significant difference in serum creatinine or estimated glomerular filtration rate (eGFR using Chronic Kidney Disease Epidemiology Collaboration 2021 equation) after the introduction of the drug, both in early stages and later in the follow-up. We found a significant reduction in albuminuria, with a reduction from 556±987 mg/g to 230±359 mg/g (p=0.004). We also noted a trend towards a reduction of glycated hemoglobin and potassium. No changes in serum uric acid, tacrolimus, cyclosporine and everolimus levels were demonstrated in our analysis. We documented one infectious adverse event (UTI), which led to the suspension of the drug. Additional information regarding our analysis is presented in Table 1.
Table 1: Comparison before and after introduction of iSGLT-2
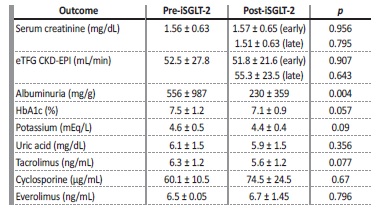
eTFG CKD-EPI: estimated glomerular filtration rate Chronic Kidney Disease Epidemiology Collaboration; HbA1c: glycated hemoglobin.
Recent studies have shown that iSGLT-2 improve both kidney and cardiovascular outcomes regardless of the presence of DM.3,5Although KTR have been excluded from large studies, it seems likely that similar benefits will be seen in this population. Notwithstanding the limitations of a small sample and the observational and retrospective nature of our analysis, we documented a significant reduction of albuminuria, absence of severe adverse events or impact on immunosuppression levels with iSGLT-2. No changes were noted in creatinine or eGFR, which could be hypothesized as due to a short follow-up or, perhaps, less marked glomerular hyperfiltration in these patients, explaining the absence of a dip in eGFR in the early stages. In agreement with published data,6 the rate of infectious complications was low. To date, there are no guidelines on this matter, but given the magnitude of DM, proteinuric kidney disease, heart failure and cardiovascular mortality in KTR, it is urgent to define which recipients may benefit from iSGLT-2 therapy.















