INTRODUCTION
Chronic kidney disease (CKD) is a progressive and irreversible condition, defined by functional and/or structural damage for at least three months, with implications for health.1 It is a global health priority that ranks ninth-leading cause of death in high income countries and accounts for one of the most prevalent diseases, estimated to affect approximately 10% of adults worldwide.2,3 Furthermore, it is responsible for a major environmental burden with high consumption of human and material resources, water, waste production, and greenhouse gas emissions.2 Growing environmental concerns have captured the attention of the nephrology community, leading to several international societies to organize and focus on a sustainable “green nephrology”. The first steps being taken on the pathway to sustainability are centered in reducing the environmental impact of hemodialysis (HD), but it is unanimous that CKD prevention and delayed progression is the most important measure to a greener nephrology.4 The last decade was particularly important in the development of new drugs to slow the progression of CKD. We review the most relevant measures of CKD prevention and the recent drugs with a proven effect on slowing CKD, combining benefits for the patients and our planet.
CHRONIC KIDNEY DISEASE PREVALENCE
CKD constitutes a major public health concern and a predisposing factor for cardiovascular (CV) disease, which collectively present a considerable burden of morbidity and mortality.5 It is estimated that the global prevalence of CKD varies from 9% to 13%, and considering the increasing ageing of world population, as well as the growing incidence of CKD risk factors, the prevalence of end-stage kidney disease (ESKD) will exponentially increase in the coming decades.6-8
In Portugal, three important population studies were conducted to assess the prevalence of CKD. The PREVEDIAB study, published in 2011, estimated a prevalence of CKD stage 3-5 of 6.1%. Despite its major importance as the first CKD prevalence study in Portugal, it did not consider the temporal criteria for CKD definition and excluded patients older than 79 years-old.9 In order to overcome its limitations, the same authors published in 2020 the RENA study, that included 3135 users of primary health care units, and estimated a CKD prevalence of 20.9%. Yet, this markedly high rate might be a result of selection bias.10 The most recent study, that included a very large cohort of 136 993 individuals, found na estimated prevalence of 9.8%, slightly higher than European prevalence rates (~7%). In this cross-section study, CKD was defined according to KDIGO guidelines and there was no upper age limit of inclusion. Furthermore, the sample size was robust and not affected by non-response bias, strengthening the validity of the estimated prevalence.11
The knowledge of the real prevalence of CKD in Portugal is extremely important given the aberrant prevalence of ESKD in Portugal. In fact, Portugal exhibits one of the highest incidence and prevalence rates of patients under dialysis treatment in Europe and worldwide.11,12 The slightly higher prevalence of stage 3-5 CKD is not the only culprit. Portugal is on the European podium of salt intake, sedentarismo and type 2 diabetes mellitus (T2DM), our population is one of the oldest in Europe and there is an easy access to HD, all importante contributors for these striking dialysis high rates.11,13,14
THE ENVIRONMENTAL IMPACT OF NEPHROLOGY
There is a bidirectional relationship between Nephrology and the environment, as both are closely intertwined and influence one another. Not only climate changes exert an impact on renal health, but also the treatment of thereof, particularly HD, is accountable for a significant ecological footprint.15 Global warming and heatwaves increase the risk of dehydration and volume depletion, enhancing the likelihood of acute kidney injury (AKI), acute-on-chronic kidney disease, and stone formation. Flooding also contributes to spreading disease vectors and water contamination, with higher risk of malaria, leptospirosis, and hantavirus, which remain among the most prevalente causes of AKI in tropical and developing countries.15-17
Although HD provides a life-saving treatment, it does have a high rate of morbimortality, reduced health-related quality of life (QoL), and entails high economic and environmental burden.4,18In a single 4-hour dialysis session, approximately 120 L of water per patient are used. In addition, the water purification process by reverse osmosis, is often inefficient and wastes up to two-thirds of the total water involved. When adding the volume needed to priming, rinsing and sterilization, the total amount of water needed per patient counts up to 500 L in a single session.17 Energy consumption, production of toxins and pollutants, as well as the waste and trash generation from consumables, are other contributing factors to the environmental impact of HD. These both directly and indirectly, lead to a significant carbon footprint estimated to be seven times higher than the average for healthcare facilities.17,19
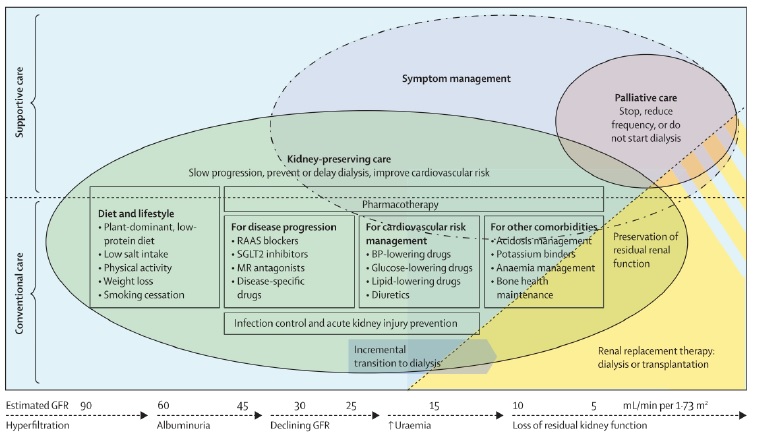
The first and perhaps most impacting measure towards a greener nephrology is to decreased the number of patients in need of renal replacement therapy.17 There are several pharmacological and nonpharmacological interventions to prevent and delay CKD that align with primary, secondary and tertiary prevention (Fig. 1).
PRIMARY PREVENTION OF CKD
Primary prevention involves taking active measures to prevent the onset of an illness. Diabetes and hypertension are the leading causes of CKD in developed countries, therefore targeting obesity, smoking cessation and alcohol consumption are part of the cornerstone of prevention.2,3Patients with established diabetes and hypertension should be routinely followed-up, as blood pressure and glycemic control has shown to prevent diabetic and hypertensive nephropathies.20
Primary prevention efforts should involve education and awareness campaigns for the general population, as well as public health approaches to tackle lifestyle changes. Encouraging a healthier lifestyle should cover physical activity and the consumption of plant-based foods over meat, reduced sodium intake, more complex carbohydrates, and less saturated fat.3 A healthier diet, decreasing meat consumption, and more active lifestyle, promoting walking and cycling is also more sustainable with significantly less CO2 gas emissions.21
SECONDARY PREVENTION OF CKD AND THE ROLE OF TELEMEDICINE
Secondary prevention relies on early detection of CKD, in order to reduce the risk of adverse outcomes such as CKD progression to ESKD, CV events, and increased mortality risk.3 The mass population screening strategy has not demonstrated to be cost-effective and is not recommended.
The focus should be on high-risk groups, including patients with diabetes, hypertension, family history of CKD, older age, and ethnic minorities and includes the screening of both the estimation of glomerular filtration rate (eGFR) and the presence of albuminuria.22,23
Unfortunately, early CKD detection is far from desirable. A recente large cohort trial that included 2.4 million CKD patients from 11 countries, found an important gap between measured CKD and diagnosed CKD. By analyzing laboratorial data from digital health care systems, including eGFR and proteinuria, and using KDIGO criteria, the authors found that 2/3 of patients with measured CKD, did not have an established diagnosed of CKD on their medical process.6
Early CKD detection and secondary prevention relies mostly in primary care units, and a close interaction between primary health care and tertiary centers is the key to success. However, primary care is not equally available to all population. This is particularly notable in Portugal, where the estimated number of citizens without family doctor exceeds one million, mostly affecting individuals residing in the geographical area of Greater Lisbon, as well as in rural areas.24
Telemedicine is an emerging and effective alternative to decentralization and might be a powerful weapon to improve general access to medical care. It reduces waiting times and financial costs, provides an improved articulation with primary care, and allows an easier contact with remote areas.25 Telemedicine is also a sustainability asset since the reduction in travel time translates into a decrease in CO2 emissions estimated between 0.70 to 372 kg CO2 per consultation.25,26
A retrospective cohort study evaluated the effect of tele-nephrology for 101 patients with CKD living in remote regions, and demonstrated that its use resulted in successful optimization of blood pressure, stabilization of eGFR and adequate control of electrolyte disturbances.27 Implementation of telemedicine may thus play a valuable tool for CKD secondary prevention with significant ecological advantages.
TERTIARY PREVENTION OF CKD
When CKD is established, the implementation of tertiary prevention measures is crucial for declining CKD progression and treat CKDrelated complications.2 For several years angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) where the few drugs with a proven benefit in retarding CKD progression and still represent first line therapy for proteinuric and non proteinuric CKD.2 In the last decade new pharmacological therapies are arousing with promising results on delaying CKD progression and the most recent guidelines from KDIGO, the American Society of Diabetes and the European Heart Association include these new drugs as first line therapy in CKD patients, with and without diabetes or heart failure.28-30 Also, several advances have been made in the drugs offered for CKD complications treatment, with a special focus on patients’ QoL.
Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers
Hypertension is a major modifiable risk factor for the development and progression of CKD and it is inherently to overactivity of the reninangiotensin system (RAS), promoting cellular injury, inflammation and fibrosis.31
RAS inhibitors, including ACEI and ARBs, decrease systemic blood pressure and promote vasodilation of renal efferent arterioles, resulting in eGFR reduction and lower intraglomerular pressure. This can effectively reduce proteinuria and glomerular basement membrane (GBM) damage, major mechanisms of CKD progression.32
Since the results of the RENAAL and the IDNT studies in 2001, which demonstrated a reduction of approximately 15% in CKD progression compared to standard of care, RAS inhibitors have become the cornerstone of treatment for proteinuric CKD and hypertensive nephropathy.22,33The hemodynamic, pleiotropic, and antifibrotic benefits of RAS inhibitors also translate in CV protection and have shown to decrease all-cause mortality.34 Despite the existence of multiple studies that prove the CV and renal benefits of RAS inhibitors, these were conducted in early CKD stages and their efficacy in advanced stages is less certain and under debate. Recently, a multi-center, randomized open-label trial enrolled 411 patients with advanced and progressive CKD, and assessed renal outcomes after RAS inhibitors discontinuation. RAS inhibitors withdrawal was not associated with slower decrease of eGFR, so it may be reasonable to maintain RAS inhibitors in advanced CKD stages.35 A larger cohort analysis that included 10 400 patients with CKD 4-5 and T2DM, also concluded that discontinuation of ACEI and ARB was associated with increased risk of cardiovascular-renal events supporting their continued use in patients with advanced CKD.36
Sodium-Glucose Co-transporter 2 Inhibitors
The sodium-glucose co-transporter 2 (SGLT2) inhibitors are regarded as a significant breakthrough in delaying CKD progression and promote CV protection.37
Although initially aimed for glycemic control in diabetic patients, it was shown that the glucose-lowering effect, was only a fraction of their overall therapeutic benefits.38 The increase in distal sodium delivery to macula densa inhibits tubuloglomerular feedback, leading to afferent arteriolar vasoconstriction, reduced hyperfiltration and proteinuria. Furthermore, natriuresis provides a moderate reduction in blood pressure, achieved even with lower eGFR levels.39 Additional hypothetical pathways through which SGLT2 inhibitors may be beneficial, include a reduction in proinflammatory and profibrotic mediators, as well as prevention of hypoxic damage by a reduction in oxygen demand.38,39
Several trials have assessed the clinical effects of SGLT2 inhibitors (Table 1).
Table 1 Kidney outcomes in clinical studies with SGLT-2 inhibitors
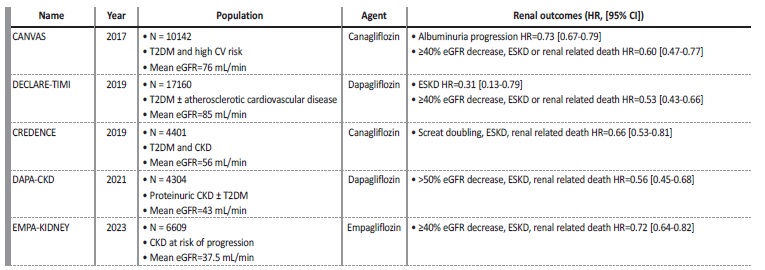
CI - confidence interval; CKD - chronic kidney disease; HR - hazard ratio; CV - cardiovascular; eGFR - estimated glomerular filtration rate; ESKD - end stage kidney disease; T2DM-type 2 diabetes mellitus.
Cardiovascular outcome trials of empagliflozin (EMPA-REG OUTCOME), canagliflozin (CANVAS), dapagliflozin (DECLARE-TIMI 58) and ertugliflozin (VERTIS-CV), have demonstrated a reduction of major CV events, mortality and hospitalization due to heart failure in diabetic patients with arteriosclerotic CV disease.39,40-43Upon conducting secondary and exploratory analysis of these trials, a benefit in patients with kidney disease has been brought into focus, revealing up to 40% reduction in the risk of progression of kidney disease.39,40-43 The CREDENCE trial was the first double-blinded, placebo-controlled randomized controlled trial that evaluated canagliflozin effects on albuminuric CKD and T2DM patients.44 The trial was prematurely stopped as a planned interim analysis demonstrated clear effectiveness for the primary outcome, with 30% reduction in the composite risk for ESKD, doubling of the serum creatinine level from baseline, or mortality from renal or CV disease.44 Later, dapagliflozin was evaluated in DAPA-CKD trial, which also included patients with albuminuric CKD, with or without T2DM, on maximal tolerated RAS inhibitors. This trial was also stopped earlier due to the efficacy regardless the presence or absence of T2DM, demonstrating a significant risk reduction in the decline of eGFR, ESKD, and mortality due to renal or CV causes.45 More recently, empagliflozin was evaluated in EMPA-KIDNEY trial, a randomized double-blinded placebocontrolled trial, that enrolled diabetic and non-diabetic patients with lower eGFR compared to the two previous renal studies. This study demonstrated a 28% reduction in progression of CKD or CV death, irrespective of the presence of T2DM.46
The benefits of SGLT2 inhibitors have been proven consistently in several large randomized clinical trials. They have showed more effective renal protection when compared to ACE inhibitors and ARBs, with a reduction of approximately 40% in CKD progression versus 15%-18%, with less hospitalizations due to hyperkalemia and AKI.25,33 Thus, SGLT-2 inhibitors represent a powerful weapon in decreasing the prevalence of ESKD, preventing hospitalizations and decreasing CV comorbidity and consequently, they might be a key-factor to sustainable nephrology
Mineralcorticoid Receptor Antagonists
Mineralocorticoid receptors hyperactivation leads to the expression of pro-inflammatory and pro-fibrotic genes that ultimately culminate in CKD and heart failure (HF).33,47A meta-analysis conducted in 2016 that evaluated the use of spironolactone or eplerenone in combination with RAS inhibitors, reported a 38.7% reduction in albuminuria compared to RAS inhibitors in monotherapy, but a significant decrease eGFR and a threefold increased risk of withdrawal due to hyperkalemia.48 Non-steroidal mineralocorticoid receptor selective antagonists, such as finerenone, have recently emerged has a safer and effective alternative. Its molecular structure is less lipophilic, allowing a homogeneous distribution between the kidney and the heart, they have a shorter half-life, no active metabolites and less side-effects.49
Two large randomized controlled trials evaluated the efficacy of finerenone in individuals with T2DM and CKD. The FIDELIO-DKD studied the role of finerenone on renal endpoints (ESKD, a sustained decrease of ≥40% in the eGFR from baseline, or death from renal causes) in patients with stage 3-4 CKD and albuminuria>300 mg/g. It revealed a reduction in the primary renal endpoints and also a significant reduction in CV events.50 FIGARO-DKD, included T2DM patients with stage 2-4 CKD and moderately increased albuminuria or stage 1-2 CKD with severely increased albuminuria and showed an overall improvement in CV outcomes when compared to placebo.51 A polled analysis from FIDELIO-DKD and FIGARO-DKD, the FIDELITY study found a relative risk reduction of 14% for the composite outcome: time to CV death, non-fatal MI, non-fatal stroke or HHF and 23% risk reduction for the composite kidney outcome: ≥57% decline in eGFR, time to kidney failure or renal death in CKD diabetic patients.52 Finenerone became part of the standard care in diabetic CKD patients, after RAS inhibitorsat maximum tolerated dose and SGLT-2 inhibitors if normal potassium levels are maintained.29
GLP-1 Receptor Agonists
Glucagon-like peptide 1 receptor agonists (GLP-1 RA) activate GLP-1 receptors on pancreatic beta-cells, enhancing insulin release and suppressing glucagon secretion and consequently lowering glucose levels. GLP-1 RA also slow gastric emptying, increase satiety and decrease appetite, making them an effective and available therapeutic option for weight loss.29,53
A 2021 meta-analysis that included T2DM and high CV risk patients, showed that GLP-1RA reduced the risk of non-fatal MI, non-fatal stroke, and CV death by 14%, and the risk of all-cause death by 12%. Regarding renal protection, there was a 21% reduction in the composite outcome of development of severely increase albuminuria, decline in kidney function, ESKD and death due to renal causes.54 This evidence placed GLP-1 RA as a second line agent in recent clinical guidelines, to improve glycemic control and decrease CV risk, in patients with CKD and T2DM beyond metformin and SGLT2i treatment.29 Nevertheless, the evidence available in real outcomes is still derived from secondary outcome analysis. The ongoing FLOW trial that compares semaglutide versus placebo, aims to assess primary kidney outcomes in subjects with T2DM and CKD, and may further clarify the role of GLP-1 RA in CKD.55
CONCLUSION
Improving patient care whilst reducing environmental burden requires a holistic approach that includes prevention on its core. Increasing awareness about CKD, promoting healthy lifestyles and early detection, are crucial to prevent and delay CKD progression. Furthermore, new pharmacotherapies are able to prolong dialysis-free time and achieve highest QoL and survival. Implementing prevention strategies and adopting environmentally-friendly practices, is the key to a better patient care and a healthier planet.