INTRODUCTION
Chronic kidney disease (CKD) patients are immunocompromised and so they have a higher risk of infection and worse clinical outcomes after coronavirus disease (COVID-19) when compared with the general population.1 Moreover, COVID-19 mortality in CKD patients is even higher than in other known high-risk groups, including patients with hypertension, obesity, chronic heart disease, or lung disease.2 The results of a large European database (ERACODA, the European Renal Association COVID-19 Database) showed that the 28-day case-fatality rate was higher in kidney transplant and dialysis patients, especially in those admitted to the hospital. Mortality was primarily associated with age and frailty in dialysis patients.3
To prevent severe disease and death, this population must be promptly immunized against severe acute respiratory syndrome coronavírus 2 (SARS-CoV-2).4 It is well established that patients with end-stage renal disease have an altered cellular and humoral immunity evidenced by the reduced response they have to several vaccines, such as the hepatitis B or influenza vaccine. However, some studies showed that the response rate is slightly higher in patients on peritoneal dialysis (PD) when compared with that of patients on hemodialysis (HD).5
There are currently several SARS-CoV-2 vaccines available and approved for use in the general population: BNT162b2 messenger RNA vaccine (Pfizer-BioNTech vaccine), messenger RNA-1273 vaccine (Moderna) adenoviral vector vaccine Ad26.COV2.S (Johnson & Johnson) and ChadOx1 nCoV-19/AZD1222 (AstraZeneca vaccine).
Recently, several studies have evaluated not only the incidence of symptomatic COVID-19 but also the humoral response in both HD and PD patients, systematically receiving two doses of vaccine against SARS-CoV-2. The third dose of the Pfizer-BioNTech vaccine has also increased antibody levels substantially in dialysis patients.6
Regarding HD patients, mRNA vaccines generated a humoral and cellular immune response in a high proportion, but also a sizable proportion of patients presented poor responses.7 Older age and immunosuppressive therapy were associated with lower antibody levels.8
Although PD patients have a reported lower prevalence of COVID-19 than HD patients (probably because of the characteristics of home-based treatment), they still have higher mortality and longer hospital admissions than the general population.3,9
A study revealed that 34 PD patients from a Unit at the Hospital Clínic of Barcelona had a significant proportion of humoral response after two doses of Pfizer-BioNTech vaccine (97%).6
Since SARS-CoV-2 vaccination is of particular importance in highrisk populations, we aimed to assess the humoral response after one and two doses of the four types of SARS-CoV-2 vaccine in PD patients.
We also aimed to identify potential factors associated with vaccine response and the prevalence of COVID-19 in our PD unit.
MATERIAL AND METHODS
We conducted a single-centre, observational, retrospective study to evaluate humoral response to vaccination in CKD patients undergoing PD.
All prevalent PD patients treated at our centre were invited to receive SARS-CoV-2 vaccination in 2020, following the prioritization by the National Health Care System. Three types of vaccine were administered: BNT162b2 messenger RNA vaccine (Pfizer-BioNTech vaccine®), messenger RNA-1273 vaccine (Moderna®), and ChadOx1 nCoV-19/AZD1222 (AstraZeneca vaccine®). All patients received vaccination according to the established schedule of the manufacturers: 21 days interval (Pfizer®), 28 days interval (Moderna®), 84 days interval (Astra-Zeneca®). The type of vaccine administered was not chosen based on any specific clinical criteria, but rather on availability and in accordance with the rules established by the National Health Care System.
The humoral response was assessed after the complete vaccination scheme had been completed (two doses) using the detection of antibodies against the two receptor binding domain (RBD) of SARS-CoV-2, the spike protein (anti-S) and nucleocapsid protein (anti-N). This was preformed using the ECLIA immunoassay (electrochemiluminescence) in Roche® COBAS e801 equipment. The cut-off value used for medical decision was the one recommended by the assay manufacturer - 0.8 U/mL.
We collected data from the medical records: age, gender, aetiology of kidney disease, comorbidities, previous immunosuppressive therapy, dialysis vintage, PD system and modality, weekly Kt/V, normalized protein catabolic rate (nPCR), albumin, type of vaccine administered, time between vaccination and antibodies detection and titre of antibodies.
Statistical analysis
Categorical data are presented as the absolute and relative number of patients. Continuous data are described with mean and standard deviation (SD) or median and interquartile range (IQR) (first quartile, third quartile), according to its normal or non-normal distribution. Fisher test was used to compare categorical parameters and the Mann-Whitney U test for continuous parameters. All statistical analyses were performed using IBM SPSS Statistics 22.
RESULTS
Population
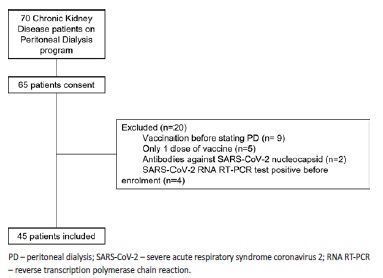
Out of our 70 patients, 45 CKD patients in the PD program were enrolled. Patients without two doses of vaccination (N=5) or vaccinated before starting PD (N=9) were also excluded, as well as patients with evidence of SARS-CoV-2 infection: SARS-CoV-2 RNA RT-PCR test positive before enrolment (N=4) or presence of anti-SARS-CoV-2 nucleocapsid antibodies (N=2). Patient selection is represented in Fig. 1.
As presented in Table 1, the mean age was 59±15 years and 26 patients were male. The three most frequent causes of kidney disease were chronic glomerulonephritis, diabetic nephropathy, and autosomal dominant polycystic kidney disease. The median dialysis vintage was 18 (3, 34) months. Five patients were under immunosuppressive therapy, and 35.6% (N=16) had diabetes.
In our population, 95.6% (N=43) received two doses of Pfizer® vaccine, one patient received Moderna® and one AstraZeneca® vaccine (Table 2).
Table 1 Baseline characteristics of the study population.
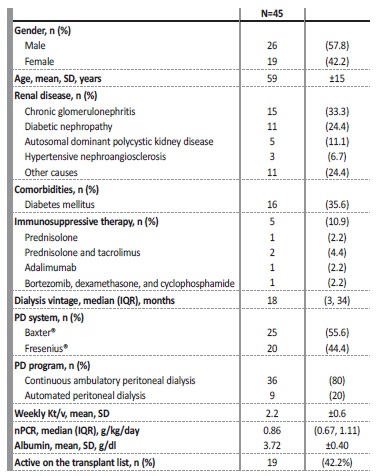
nPCR - normalized protein catabolic rate; PD - peritoneal dialysis; SD - standard deviation
The median time between vaccination and evaluation of humoral response was 7.2 (7.1, 7.6) months. At the evaluation, 95.6% of the patients developed a positive antibody response, according to the assay-specific cut-off value of 0.8 U/mL for anti-spike antibodies. The median anti-spike antibodies value was 92 (36, 447) U/mL.
Only two (4.4%) patients remained seronegative: a patient submitted to kidney transplant in 2017, who started kidney replacement therapy four months before vaccination and was medicated with prednisolone and tacrolimus, and another patient with psoriasis, treated with adalimumab. The previous history of immunosuppressive therapy was associated with no response after two doses of vaccine (100% response in non-immunosuppressed vs 60% in immunosuppressed patients, p=0.010). No other studied variable was associated with poor response to vaccination.
After vaccination, only two patients were diagnosed with symptomatic SARS-CoV-2 infection with mild symptoms. There were no hospital admissions or deaths caused by SARS-CoV-2 infection.
DISCUSSION
PD may be a favourable technique to prevent the spread of the infection, as it is a home-based therapy with the ability to self-isolate appropriately. Patients are seen less frequently, limiting the close interaction between patients and healthcare workers who may contract and spread the disease.10 The advantages of home-based therapy go beyond the mitigation of the risk of infection; in addition, it allows management of patients at the expense of a lower demand of human, economic and material resources. Indeed, there are remote devices that allow the clinical staff to assess treatment at home and make changes, when necessary, like the Share Source program of Baxter®.
A study found that the incidence of symptomatic COVID-19 inpatients on PD was close to that of the general population in the same city.11 In fact, from our cohort (70 patients on the PD program), only four patients (5.7%) had been infected by SARS-CoV-2 before vaccination (with mild symptoms and no need for hospitalization).
Furthermore, most of the patients successfully developed antibodies against SARS-CoV-2 (95.6%) after vaccination and no significant side effects have been reported. PD patients may reach better protective antibody titres than those of HD patients.5 This conclusion can be related either to the technique itself or to the presence of a higher residual renal function, which was not analysed in this study.
This data reinforces the idea that this population should be vaccinated as soon as possible as most of them seroconvert and are therefore likely to be protected from severe COVID-19.
As in other studies, we also found that the use of immunosuppressive therapy is a predictor of weak response in comparison to nonimmunosuppressed patients. Two patients (4.4%) that were taking prednisolone and tacrolimus and another adalimumab were weak responders after the second dose. For this reason, the administration of a third vaccine dose was firstly proposed for patients taking immunosuppressive medication.12
Important limitations of our study were the small cohort size and the measurement of only the humoral response to vaccination, without considering the cellular response. Moreover, we did not compare our results to a control group (PD patients that had not received the vaccine).
The available data for this retrospective, observational, analysis only included one timing of evaluation of humoral response to vaccination; we lack information on duration and persistence of the response and protection. Additionally, we did not study the association between the reported level of humoral response to vaccination and the actual risk of developing infection (as well as its severity). Both of these topics would have been relevant to address, and further investigation is needed to study them.
In conclusion, even though real-life clinical protection from COVID-19 in CKD patients undergoing PD is yet to be seen, we found that immunization programs were effective in generating a humoral response. These results support that SARS-CoV-2 vaccination is of special significance in high-risk immunocompromised populations such as patients on PD. Further studies are warranted to evaluate the long-term maintenance of immune responses to SARS-CoV-2 vaccination.