INTRODUCTION
Peritoneal dialysis (PD) patients have an increased risk of developing abdominal wall complications, such as hernias and dialysate leaks, due to the increased intra-abdominal pressure generated by the
amount of dialysis fluid.1
Abdominal wall hernia is a common mechanical complication of PD, with reported prevalence rates ranging between 7% and 37%.2,3,4,5 Besides its impact on the patient’s body image and the pain associated, a hernia might have a negative repercussion on dialysis clearance, ultrafiltration, in case of dialysate sequestration, and, consequently, on technique survival.4-6
It is essential to identify risk factors for hernia formation and take preventive measures to reduce their incidence in PD patients. Previous studies have identified several risk factors, such as multiparous females, autosomal dominant polycystic kidney disease (ADPKD), low body weight, larger dialysate infusion volume, previous abdominal surgeries including hernia repair.4-10
Few studies have assessed the influence of hernias on PD and patient’s survival with conflicting results. Yang et al reported a higher risk of PD withdrawal after hernia development 5, however other studies have shown no differences regarding technique outcomes.11,12
The aim of our study was to determine the incidence of hernia in PD patients, identify possible risk factors for its formation and evaluate hernia impact on technique survival.
MATERIAL AND METHODS
We conducted a single-center retrospective cohort study including all prevalent PD patients in our unit between January 1st 2010 and October 31st 2020. Patients with a diagnosis of hernia after PD initiation constituted the hernia cohort under study and were compared to control cohort (without hernia formation during PD therapy).
The study protocol was according to the Declaration of Helsinki. The collection of clinical information for this study was approved by the Ethics Committee of the Hospital Centre under the number 135/2022. Given its retrospective and non-interventional nature, we did not obtain the patients’ informed consent.
Data was collected from patients’ electronic record and included baseline characteristics such as age, sex, body mass index (BMI), cause of end-stage renal disease, diagnosis of diabetes mellitus (DM), hypertension (HTN), ischemic heart disease, peripheral vascular disease, cerebrovascular disease, smoking habits, chronic obstructive pulmonary disease, previous abdominal surgeries (excluding surgical procedures related to hernias), number of pregnancies and caesarean births, and history of hernia before dialysis initiation. The PD modality used one month after starting PD (continuous ambulatory PD (CAPD) or automated PD (APD)) was analysed. We included patients on APD with either a daytime dwell or a daytime dry abdomen. For patients who developed a hernia while on maintenance PD, the dialysate exchange volume adjusted to body surface area used at the time of diagnosis was recorded; to compare this variable to the control cohort, the maximal volume used at any point of PD treatment, adjusted to body surface area, was reported.
Categorical variables are presented as frequencies and percentages and were compared with the use of Fisher’s exact test or the chi-square test, as appropriate. Continuous variables are presented as means and standard deviations, or medians and 25th and 75th percentile for variables with skewed distributions. Normal distribution was checked using Shapiro-Wilk test or skewness and kurtosis. Continuous normally distributed data variables were compared with the use of Student’s t-test and continuous non normally distributed data were compared with Mann-Whitney test. Logistic regression was performed to find predictors of hernias in PD patients. Kaplan-Meier estimates were used to evaluate survival curves for peritoneal dialysis technique. All reported p values are two-tailed, with a p value inferior to 0.05 indicating statistical significance. Statistical analyses were performed by SPSS for Windows software version 26.0 (SPSS Inc., Chicago, IL).
RESULTS
A total of 155 patients were included, 58.7% (n= 91) males, with a mean age of 55.9 ± 13.5 years, 21.3% (n= 33) were diabetic and 73.5% (n=114) were hypertensive, with a median PD vintage of 25.0 months (14.0-67.6). During the study period, a total of 54 hernia events and 32 hernioplasties were registered during PD in 45 patients (29% of the total population). The overall incidence rate was 0.09 hernias/patient/year. The types of hernia were umbilical in 35 cases (64.8%), inguinal in 14 (25.9%), incisional in 4 (7.4%) and epigastric in 1 (1.8%).
The mean time from the start of PD to the development of a hérnia was 14.2 ± 12.0 months. Table 1 illustrates the comparison between the patients with and without hernia (control group). Compared to the control group, the hérnia cohort had a significantly greater proportion of patients with ADPKD (24.4% vs 6.4%, p=0.001). This cohort had a higher frequency of previous abdominal surgeries (44.4% vs 11.8%, p<0.001) and previous surgically treated hernias (22.2% vs 5.5%, p=0.003). They were more frequent on CAPD (82.2% vs 61.8%, p=0.014) and in the patients that used a larger total volume of dialysate (median of 3.9 vs 3.6 L, p=0.001). The remaining baseline characteristics were similar between groups, including age, sex, BMI, DM, HTN and cardiovascular disease. The daytime infusion volume was not associated with a greater incidence of hernias (p=0.401). The number of pregnancies and cesarean births did not differ significantly between groups (p=0.477 and p=0.057, respectively).
Table 1 Comparison between clinical characteristics of peritoneal dialysis patients with and without hernia.
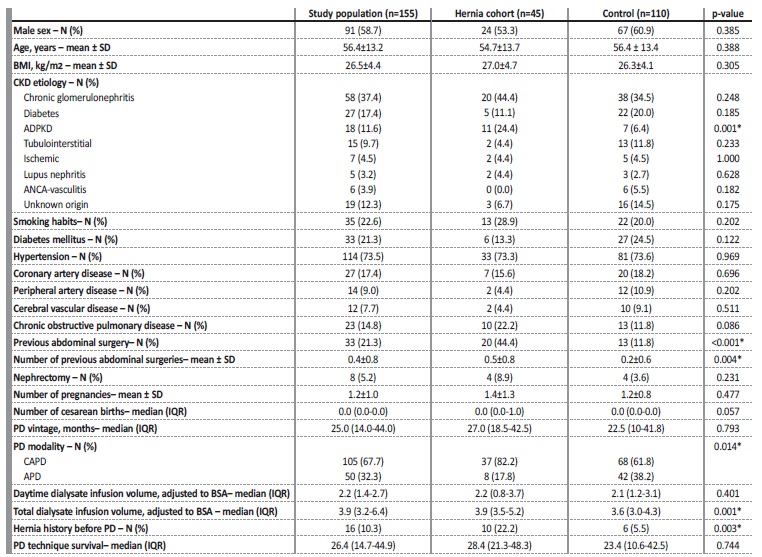
ADPKD - autosomal dominant polycystic kidney disease; APD - automated peritoneal dialysis; BMI - body mass index; BSA - body surfaced area; CAPD - continuous ambulatory peritoneal dialysis; CKD - chronic kidney disease; IQR - interquartile range; PD - peritoneal dialysis; SD - standard deviation; *p<0.05
When logistic regression was applied, a diagnosis of ADPKD [OR (odds ratio) 3.925; 95% confidence interval (CI), 1.208 - 12.750], history of surgical repaired hernias (OR 5.586; 95% CI 1.540 - 20.255)
and a higher number of previous abdominal surgeries (OR 1.680; 95% CI 1.025 - 2.752) were independent risk factors for the development of a hernia during PD (shown in Table 2).
Table 2 Logistic regression for predictors of hernia development in PD patients
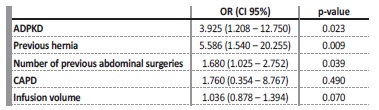
ADPKD - autosomal dominant polycystic kidney disease; CAPD - continuous ambulatory peritoneal dialysis; CI - confidence interval; OR - odds ratio; PD - peritoneal dialysis
A total of 32 hernias underwent surgical treatment, with 27 (84.3%) of these being elective procedures. Three of them underwent tensionfree mesh hernia repair, and the other 24 were managed with a hérnia repair surgery without mesh. These elective surgeries occurred within a median of 70.0 days following referral. In contrast, five cases required emergency surgery due to incarcerated hernia (n=4) and strangulated hernia (n=1). The remaining patients were placed under conservative management while on waiting-list for surgery. In 21 cases, low-volume PD was started postoperatively, within a mean of 3.8 days after the hernia repair. The remaining 9 patients were transferred to hemodialysis immediately after surgery. Of these 8 patients returned to PD, within an average of 52.1 days after the procedure, and 1 patient was permanently transferred to hemodialysis.
Six hernias (18.8%) recurred, with a mean time to recurrence of 14.8±11.1 months (range: 3.5 - 29.6 months). A higher daytime infusion volume was associated with hernia recurrence (p=0.045).
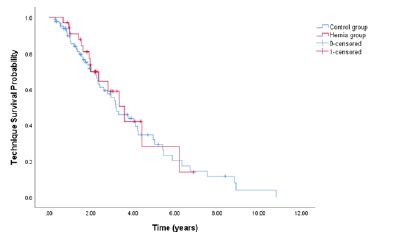
At 1, 2, and 3 years, PD technique survival censored for death and transplantation was, respectively, 86.7%, 53.3%, and 28.9% in the hérnia cohort and 75.5%, 50.9%, and 36.4% in control subjects. In Cox-regression model, there was no statistically significant difference in long-term technique survival between the groups (p=0.700, shown in Fig. 1).
DISCUSSION
Abdominal hernias are more frequent in PD patients compared to healthy subjects. The prevalence rate in our study was 29% and the incidence rate was 0.09 hernias per patient per year, which is comparable to previous published series.4-6Umbilical hernias were The most common subtype, followed by inguinal hernia.
We found that autosomal dominant polycystic kidney disease, history of surgical repaired hernias and a higher number of previous abdominal surgeries were risk factors for hernia development in PD patients. All of them are patient-related factors, not related to PD itself.
Increased intra-abdominal pressure due to polycystic kidneys have been postulated as being responsible for hernia formation.4,13-15
However, there are no consensus that ADPKD constitutes an independente risk factor for hernia formation in PD patients.5 In the presente study, 60% of patients with ADPKD had developed abdominal hérnia and multivariate logistic regression analysis showed that ADPKD was an independent risk factor for the development of hernia.
Surgical repaired hernias prior to PD start were a risk factor for hernia development during PD treatment. These patients have na abdominal weakness even before having increased abdominal pressure due to the dialysate. Besides some surgical techniques are associated with higher recurrence rates and the sutures holding the abdominal muscles become less effective with time, which can justify our result.
Previous abdominal surgeries were associated with a higher incidence of hernias. In our study, only 7.4% were incisional hernias with majority (64.8%) being umbilical hernias. Banshodani M et al also found a higher proportion of patients with previous surgery in patients with umbilical hernias (versus the control group), although in the logistic analysis it was not an independent risk factor.9 To our knowledge, no prior studies have found an explanation for this association.
Another potential risk factor for hernia formation is the use of large volumes of dialysate, which can increase intra-abdominal pressure.7,16 However, the correlation between intra-abdominal pressure and hernia risk in PD patients remains controversial.5,13In our study, although we did not directly measure intra-abdominal pressure, we observed that, despite the hernia group having a higher total volume of dialysate compared to the control group, after the logistic regression analysis, a higher maximal dialysate volume was not a predictor of hernia development. It is important to note that intraperitoneal pressure is influenced by several factors, and increased intraperitoneal volume is just one among them. Other factors, including the infusion rate and dwell time of the fluid, the PD vintage, the patient’s body position and individual patient characteristics (such as body weight) also play a role in intraperitoneal pressure.1,7,8,17
Despite PD modality per se had not been shown to be an independente risk factor for hernia formation, we found that patients on CAPD experienced more hernias than those on APD, which is consistente with earlier research.4,6Gloria del Peso et al showed that patients receiving CAPD experienced more hernias than individuals using only cycler.4 In this study, the authors found that the largest fill volume used by both groups of patients (CAPD and APD) was similar. When only the daytime fill volume was examined, CAPD patients significantly required much higher volumes than APD patients. In our study, we did not compare the maximum or daytime infusion volumes between the two PD modalities. Since both works found a higher incidence of hernias in CAPD, it can be hypothesized that the higher-pressure presente in the abdominal cavity while the patient is standing or sitting may be in part responsible for hernia development.7,16
The high rate of hernias in our PD population emphasizes the importance of an exhaustive search for hernia in all PD patients, especially in those with ADPKD, who may have had prior abdominal surgery or a hernia at baseline. The option for APD treatment can minimize the development of this mechanical complication in these high-risk patients.
Only 3% of the patients who underwent surgery in our study had to switch permanently to hemodialysis. Our recurrence rate of 18.8 is higher than previously reported in prior studies.6,18-20 This finding may be explained by the low number of cases that were managed with a tension-free mesh repair. This surgical technique has been reported as safe for PD patients and associated with lower recurrence rates, from 0% to 10%.6,20,21 We found that larger daytime infusion volumes are associated with hernia recurrence following surgical repair, which supports the use of APD with low daytime Exchange volumes in those patients to avoid recurrences.
One observational study found that the development of a hérnia during PD therapy is associated to an 15% increased risk of PD discontinuation.5 The impact of this mechanical complication on PD technique survival is multifactorial.5,6,12,19 It can compromise the adequacy of PD treatment, affecting its overall effectiveness and potentially leading to technique failure. When surgical repair is necessary, during the recovery time definitive transition to hemodialysis may occur, further influencing technique survival. Additionally, there is a risk of hernia recurrence after surgical repair, which may require additional surgeries and result in further interruptions to PD treatment, impacting technique survival. Patient compliance and the ability to manage self-care tasks, including proper hernia care, can also influence PD technique survival.
Our study did not find any evidence that hernia formation had a negative impact on drop-out-rates. These finding may be attributed to timely surgical correction in majority of cases and a thoughtful approach to the choice of PD modality.
This study presents some limitations since it is a single-center, retrospective study, and data on other potential factors influencing the outcomes could be under evaluated. Adequacy of PD, intraperitoneal pressure, residual renal function, nutritional status, infectious complications related to PD and quality-of-life data were not assessed.
In summary, we found that ADPKD, previous abdominal surgery and previous hernia were independent risk factors for the development of abdominal hernia and that development of this mechanical complication had no impact on PD technique survival. Prompt surgical intervention, transition to APD with low volumes during daytime period, careful management of the hernia, and close monitoring of the patient’s PD treatment are crucial to minimize disruptions and complications that may affect technique survival. Individualized management plans should be developed to ensure the best possible outcomes for PD patients with abdominal hernias.