Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Nascer e Crescer
versión impresa ISSN 0872-0754versión On-line ISSN 2183-9417
Nascer e Crescer vol.27 no.3 Porto set. 2018
https://doi.org/10.25753/BirthGrowthMJ.v27.i3.10562
CASE REPORTS | CASOS CLÍNICOS
Spondylodiscitis in pediatric age – a diagnostic challenge
Espondilodiscite em idade pediátrica – um desafio diagnóstico
Joana FerreiraI, Marta AlvesI, Alícia RebeloI, Teresa São SimãoI, Cláudia TavaresI, Cristina FerreiraI
I Department of Pediatrics, Hospital Senhora da Oliveira – Guimarães. 4835-025 Guimarães, Portugal. joanaferreira.med@gmail.com; martasfariaalves@gmail.com; aliciarebelo88@gmail.com; teresinhaped@gmail.com; anapediatra@gmail.com; crispediatra@gmail.com
Correspondence to
ABSTRACT
Spondylodiscitis is an inflammatory process of the intervertebral disc and the adjacent vertebral endplates and mainly involves the lumbar spine. Clinical suspicion is not raised in most instances, often resulting in difficult and delayed diagnosis. The onset may be insidious and clinical signs can be mild and unspecific. This is also true with laboratory tests, which often remain within the normal range. Refusal to walk and back pain are the main symptoms, and magnetic resonance imaging of the spine is the gold standard for the diagnosis. The duration and type of treatment are controversial, but the use of antimicrobial therapy together with rest and immobilization showed good results in specific cases, leading to a progressive recovery.
We describe a previously healthy two-year old boy with spondylodiscitis, in whom no direct infectious pathogen was identified. We discuss the clinical features, laboratory findings, as well as the outcome of this clinical entity based on a review of the reported cases.
Keywords: Back pain; discitis; spondylodiscitis; vertebral infection
RESUMO
A espondilodiscite é um processo inflamatório do disco intervertebral e da superfície dos corpos vertebrais com predileção pela região lombar.
Do ponto de vista clínico e laboratorial é uma doença com achados inespecíficos, o que pode resultar em dificuldades e atrasos no diagnóstico se não existir uma elevada suspeição clínica. A recusa da marcha e a dor lombar são os principais sintomas, sendo a ressonância magnética da coluna o ideal para o diagnóstico. A etiologia e o tratamento ainda não são consensuais. O uso de antibióticos, juntamente com o repouso e a imobilização da coluna vertebral têm-se associado, na maioria dos casos, a uma evolução clínica favorável.
Apresenta-se o caso de uma criança com dois anos de idade, previamente saudável, com espondilodiscite sem agente patogénico direto identificado e discute-se a apresentação clínica, as alterações nos exames laboratoriais, bem como a evolução desta entidade com base numa revisão da literatura.
Palavras-chave: Discite; espondilodiscite; infeção vertebral; lombalgia
INTRODUCTION
Pyogenic infection of the spine was first reported by Lannelongue in 1879, however spondylodiscitis was not defined as an independent entity until 1925, by Mayer.1,2
The term spondylodiscitis covers vertebral osteomyelitis, spondylitis and discitis, which are different manifestations of the same pathological process.1-5 It is an uncommon disorder with an estimated incidence ranging from one to two cases per 32.500 pediatric hospital evaluations per year.1-6 The average age for the diagnosis in children is approximately two to eight years, although some cases can occur in adolescence.1,7-9 The etiology is still debated in literature and various pathogenic factors have been identified.1-5,10,11 From a clinical point of view, it is a disease with unspecific signs and symptoms, sometimes resulting in a difficult and delayed diagnosis. In addition, laboratory investigations are often unhelpful and blood cultures are negative in approximately 50% of patients.12 The long-term outcome usually is good.1,3,7,13
The authors report a child affected by spondylodiscitis who was successfully treated with antimicrobial therapy. This clinical report reinforces the need to consider this diagnosis when a child refuses to walk.
CASE REPORT
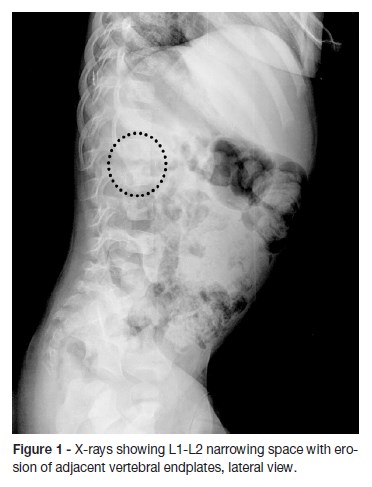
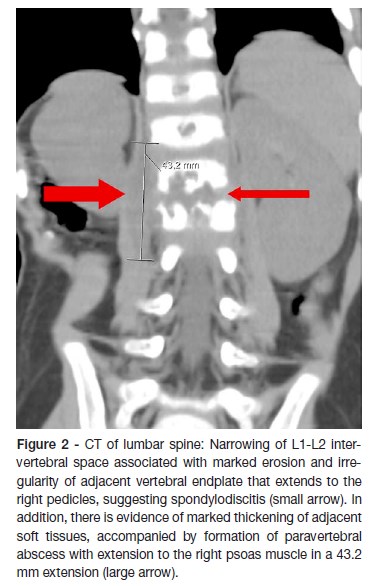
A two-year old boy was born at term after an uncomplicated pregnancy and a normal delivery. The family history was unremarkable. He was brought to the emergency department with a two-day history of irritability, abdominal pain and a reluctance to sit and walk. The parents stated that about two months before admission the boy has had a foot sprain at school (the level of injury was unclear) which was treated at home with rest and paracetamol. A week before the admission, the boy started nasal congestion, cough and decreased appetite. On physical examination, the infant had refusal to flex the spine. He was afebrile with normal vital signs and had no neurological dysfunction. No lumbar lesions or regional lymphadenopathy were appreciated. A complete blood count revealed 10200 white blood cells (WBCs)/uL with 30,2% neutrophils, 61,1% lymphocytes, 1,3% eosinophils and 6,8% monocytes; hemoglobin level at 10,6 g/dL; and platelet count at 587000/uL. Hepatic function panel and urine analysis were normal. Erythrocyte sedimentation rate (ESR) was increased at 32mm/hour (reference values between 0–10mm/hour). The other laboratory tests, including C-reactive protein (CRP) serum concentration were within normal range. Chest radiography was normal. Lumbosacral spine radiography showed a reduction of disc space with erosions of adjacent vertebral endplates at L1-L2 (Figure 1). He was hospitalized for further etiological investigation and treatment after suspicion of spondylodiscitis diagnosis. Computed tomography (CT) (Figure 2) and magnetic resonance imaging (MRI) of the spine (Figure 3) showed diffuse L1-L2 intervertebral disc and the adjacent vertebral body infiltration consistent with spondylodiscitis, accompanied by formation of paravertebral abscess with extension to the right psoas muscle. Empirical treatment with intravenous ceftriaxone and flucloxaciline was started.
Serum antibodies, anti-Herpes Simplex Virus 1 and 2, Adenovirus, Cytomegalovirus, Mycoplasma pneumoniae, Borrelia burgdorferi, Brucella melitensis and Epstein–Barr virus and blood culture results (obtained from two samples) were negative.
Mantoux tuberculin skin test and Interferon-Gamma Release Assays (IGRA) blood test for tuberculosis were equivocal (first one was nonreactive and second one was indeterminate). Polymerase chain reaction (PCR) for M. tuberculosis from early morning gastric washing samples was negative. Pathology staining for acid-fast bacilli and cultures for mycobacteria were also reported as negative.
After the first week of treatment the child had no abdominal or spinal pain and laboratory tests showed a decrease in the ESR value to 3mm/hour, with the level of WBC count and CRP remaining within normal range. Two weeks later the patient began to sit and to walk. After three weeks he had a full clinical recovery, the inflammatory markers were normal and intravenous treatment was then switched to oral for another three weeks. Bed rest was carried out and combined with the administration of antibiotics until evidence of clinical and laboratory resolution of the condition.
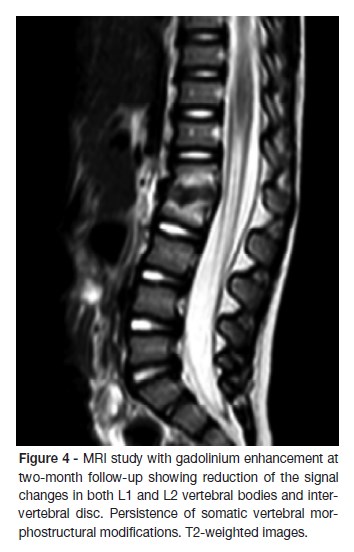
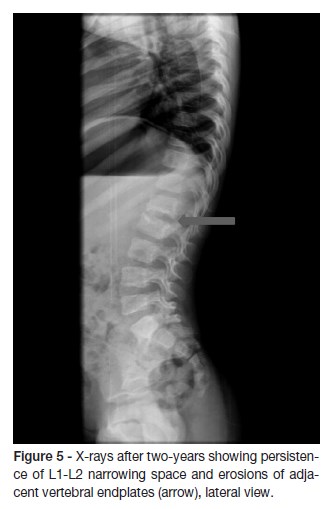
At the first month follow-up, IGRA test was repeated with negative result. At the two-month follow-up, a new spinal MRI showed a reduction of the signal changes in both L1 and L2 vertebral bodies and intervertebral disc, despite the persistence of somatic vertebral morphostructural modifications (Figure 4). X-rays taken after two-years showed persistence of segmental reduction at L1-L2 but with no functional significance (Figure 5). The child asymptomatic and presents a normal neurological examination.
DISCUSSION
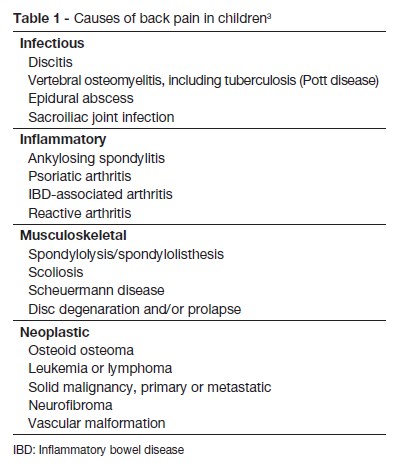
This case has highlights the importance of clinical suspicion in the early diagnosis of spondylodiscitis an insidious disorder, not always accompanied by specific laboratory findings. However it is important to reinforce the need to consider the diagnosis every time a child suddenly refuses to walk, crawl or stand up. In fact, Brown et al reported a series of 11 consecutive cases where those signs were present in 63% of patients.14 Back pain is also often reported. Fever is usually low grade or absent and abdominal pain may be the predominant complaint in lesions involving L1, as occurred in this case.12,15,16 When the cervical spine is affected, manifestations may include dysphagia and stiff neck.16 Contracture and spasm of paravertebral and psoas muscles occur with limited mobility of the spine, which can be extremely painful and cause loss of the lumbar lordosis.3,10,12,14,16,17 Other findings may include percussion tenderness over the involved spine, decreased muscle strength or reflexes or ileus (with high lesions: T8-L1). Unspecific symptoms are also common such as fatigue, irritability, loss of appetite, and a lack of desire to play.13,16 Table 1 shows other causes of back pain in children, which have different implications for treatment or prognosis, and must also be considered. A review of the literature of spondylodiscitis in children suggests that it has a biphasic age distribution primarily affecting the young toddler, with a second peak in adolescence.9,15 The pathophysiology of the condition has not been clearly established. Trauma, inflammation and infection have all been implicated.3,18,19 The pathogens can infect the spine from three sources: by haematogenous spread (arterial or venous), by external direct inoculation, or by contiguous spread from adjacent tissues. The predominant form is blood haematogenous. Unlike adults, the intervertebral disc in young children is vascularized.13,20 Vascularization in children is made up of vessels across the cartilaginous vertebral plate and into the ring; after eight years of age, these vessels disappear, but a rich anastomotic network of vessels remains in the periphery of the disc. The vascularized nature of the intervertebral disc in children explains the higher incidence of this disease in this age group. According to various studies, this fact explains the hypothesis of bacterial etiology, and also the satisfactory response to treatment with antibiotics.7,12,18,20,21 In tuberculosis, the destruction starts with the vertebral body and spreads to the adjacent vertebra through the ligaments. Extensive bone infarcts lead to the formation of cavitation and compression fractures. Failure to control the infection can cause paravertebral, psoas or epidural abscess.16 Pyogenic spondylodiscitis from the haematogenous source particularly affects the lumbar spine, followed by the thoracic and cervical spine, reflecting the volume of blood flow.3,6,22 In tuberculosis, we observed involvement of the thoracic spine.16
We noticed that the patient had an episode of trauma two months before the development of spondylodiscitis which could have been implicated in the etiology for the condition as well as explain the presence of spinal radiographic changes at the time of the diagnosis (usually not seen until second or third week of onset of the disease). On the other hand, the presence of a prodromal illness one week before the admission to the hospital could lead to believe in the infectious nature for the process. The absence of a marked systemic response could have simply reflected an appropriate local host response to a pathogen of low virulence, such as Kingella kingae.
When reviewing the laboratory investigations, we found that the WBCs and CRP values were normal, but ESR had increased above 30mm/hour. In fact, ESR is usually elevated in ≥90 percent of patients.1,4,15,23
As seen in other reported cases, blood cultures were negative and thus unable to provide sufficient etiologic information. In series where positive cultures were present, Staphylococcus aureus was the most common isolated organism.1,6,22,24 Recently the reported number of cases of K. kingae infection has markedly increased. Many studies have demonstrated that this pathogen has become the leading cause of spondylodiscitis in children aged between 6 and 48 months and the microorganism is currently recognized to account for 30% to 93.8% of all culture-positive osteoarticular infection.24,27 The high rate of sterile blood cultures and the frequent failure to identify the causative pathogen, even on disk or vertebral aspiration, is another very suggestive argument that most cases of the infantile form of spondylodiscitis are due to K. kingae. In fact, the recovery of K. kingae from purulent specimens seeded onto solid culture media is suboptimal and results are negative in a frustrating proportion of cases.23 Thus sterile blood cultures can be interpreted as false-negative results correlated to inappropriate techniques of K. kingae identification in spondylodiscitis. In our case, we could not prove that the spondylodiscitis was actually caused by K. kingae, as we did not made specific culture nor used molecular detection methods. We also did not perform any biopsies or needle aspirations for diagnostic purposes which reflected our risk/benefit analysis based on the low rate (less than 50%) of positive cultures reported in previous series.7,9 According to some authors, this procedure should be reserved for children not responding to intravenous antibiotics.7
Mycobacterium and Brucella infection were also investigated. Brucellosis still has a high incidence in many parts of the world, including the Mediterranean littoral and remains an important cause of granulomatous spondylodiscitis.25,28 It should be noted that in the early stages, the differential diagnosis between unspecific infection (pyogenic discitis) and granulomatous infection (spinal tuberculosis or brucellosis) can be difficult. In this case, patients clinical improvement with empiric antibiotic therapy led us to exclude the tuberculosis hypothesis.
We used CT and MRI to establish the diagnosis. MRI has high sensitivity (96%) and specificity (94%), and remains the gold standard complementary imaging method for the investigation of pyogenic infection of the spine, particularly in the early stages.1,7,15,17,22,26 MRI can also exclude alternative diagnoses such as vertebral osteomyelitis and tumors.1,22
In our case, changes such as reduced disc space and erosion of the end-plate were evident in spine radiography, suggesting that the disease had been present for at least two to three weeks (Figure 1).7,9
Bone scintigraphy with technetium-99m pyrophosphate shows positive signs within one to two days of the onset of the infection. The drawbacks of the technique however include low specificity and insufficient spatial resolution of the affected site. Moreover, the high accuracy of the MRI has led to scintigraphy falling into disuse.10,13,27 Computed tomography (CT) scan is easy to perform, faster and more affordable than MRI. The areas of erosion of the endplates that appear early in the affected vertebral levels are easier to detect by CT scan than by the images from plain radiographs.10,13,27
Management for spondylodiscitis is not standardized. Some retrospective data suggests that initial treatment with intravenous antibiotics until the child shows clinical improvement followed by oral antibiotics is associated with a somewhat earlier response and fewer relapses than treatment with analgesia alone.1,4 Due to this, our hospital routinely prescribe antibiotics as a first-line treatment for the condition. The recommended empiric antibiotic therapy is a combination of a third-generation cephalosporin combined with an antistaphylococcal agent.1,14,21 Immobilization, either through bed rest, or occasionally, bracing or casting, may assist with pain control. In our case we favor immobilization through bed rest; no body plaster or orthosis was used. Restricted activities should be maintained for 10–12 weeks, or until evidence of clinical and laboratory resolution.6,12 Treatment with antibiotics and rest was efficacious in this case leading to clinical resolution and recovery in a few weeks.
Recommendations regarding the duration of treatment, varies between institutions, ranging from one week to three weeks intravenously, followed by supplementary oral therapy until resolution of the inflammation and clinical improvement.3,4,12,15,16,27 The total treatment can last from two weeks to six months, according to the patients response.3,7,10,14,21 In our case we used a combination of regular clinical assessment and serial measurement of inflammatory markers as a guide to the duration of treatment. The criteria for the discontinuation of the antimicrobial treatment included resolution of the symptoms and normalization of ESR e CRP. The ESR usually increases during the first few days of treatment and then declines slowly in the follow weeks. CRP returns to normal much more rapidly than ESR and it has been proposed that a weekly reduction of 50% in CRP represents favorable evolution.3,10,16,27 We also used MRI for monitoring the therapeutic response during the course of spinal infection, however we recognize that MRI scans ordered in patients responding well clinically often gives conflicting results and that we should be cautious in interpreting follow-up MRI images.24 Persistent or increased gadolinium enhancement seen in the context of clinical improvement does not necessarily represent deterioration or treatment failure, as seen in this case.
Surgery is rarely indicated for disc infection in children. It may be necessary in cases of neurological deficit due to medullary or root compression, major destruction of the vertebral bodies, progressing to rapid transformation of the deformity into kyphosis, spinal instability, or failure of conservative treatment.9,12,14,16,20
The outcome is usually good, although anomalies of the disc space and adjacent vertebrae (often asymptomatic) are common findings, as reported in 50 out of 55 patients in two series.1 In our case, radiographic assessment at two years follow-up, showed persistent abnormalities but no restriction of spinal movements. As a result, we believe that long-term follow-up is necessary for all children with this disorder.
CONCLUSION
In summary, spondylodiscitis in children is an uncommon disorder and diagnosis is challenging since symptoms and signs are often insidious and nonspecific; refusal to walk and back pain are the most common.2 The etiology and the best treatment are still under debate. Blood cultures are usually negative, and identification of the causative pathogen can be challenging.6 Radiographic changes may only be seen within two to three weeks after the onset of the disease and spine MRI remains the gold standard to confirm the diagnosis.7-9,15,16,29 This case highlights the importance of clinical suspicion and early diagnosis to reduce the risk of negative outcomes.
REFERENCES
1. Nigrovic PA. Back pain in children and adolescents: causes [consultado em 01 de dezembro de 2016]. Disponível em https://www.uptodate.com. [ Links ]
2. Oliveira SKF. Dor e infecção. Reumatologia para Pediatras. Revinter. Rio de Janeiro 2003: 245-82. [ Links ]
3. Early SD, Kay RM, Tolo VT. Childhood diskitis. J Am Acad Orthop Surg 2003; 11:413-20. [ Links ]
4. Ring D, Johnston CE 2nd, Wenger DR. Pyogenic infectious spondylitis in children: the convergence of discitis and vertebral osteomyelitis. J Pediatr Orthop 1995; 15:652–60. [ Links ]
5. Rocco HD, Eyring EJ. Intervertebral disk infections in children. Am J. Dis. Child. 1972; 123:448-51. [ Links ]
6. Fucs P, Mevez R, Yamada H. Spinal infections in children: a review. Int Orthop. 2012; 36:387–95. [ Links ]
7. Brown R, Hussain M, McHugh K, Novelli V, Jones D. Discitis in young children. J. Bone Jt. Surg 2001; 83:106–11. [ Links ]
8. Carty H. Radionuclide bone scanning. Arch Dis Child 1993; 69:160-5. [ Links ]
9. Fernandez M, Carrol CL, Baker CJ. Discitis and vertebral osteomyelitis in children: an 18-year review. Pediatrics 2000; 105:1299–304. [ Links ]
10. Garron E, Viehweger E, Launay F, Guillaume JM, Jouve JL, Bollini G. Nontuberculous spondylodiscitis in children. J. Pediatr. Orthop. 2002; 22:321–8. [ Links ]
11. Rubio B, Calvo Rey C, Garcia-Consuegra J, Ciria L, Navarro ML, Ramos JT. Spondylodiscitis in the autonomus community of Madrid (Spain). An Pediatr (Barc) 2005; 62:147–52. [ Links ]
12. Skaf GS, Kanafani ZA, Araj GF, Kanj SS. Non-pyogenic infections of the spine. Int. J. Antimicrob. Agents. 2010; 36:99–105. [ Links ]
13. Kayser R, Mahlfeld K, Greulich M, Grasshoff H. Spondylodiscitis in childhood: results of a long-term study. Spine 2005; 30:318–23. [ Links ]
14. Dormans JP, Moroz L. Infection and tumors of the spine in children. J Bone Joint Surg Am. 2007; 89(Suppl 1):79-97. [ Links ]
15. Cushing AH. Diskitis in children. Clin Infect Dis. 1993; 17:1–6. [ Links ]
16. Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: Update on diagnosis and management. J. Antimicrob. Chemother. 2010; 65 Suppl 3:iii11-24. [ Links ]
17. Gabriel KR, Crawford AH. Magnetic resonance imaging in child who had clinical signs of discitis. Report of a case. J Bone Joint Surg Am 1988; 70:938-41. [ Links ]
18. Crawford AH, Kucharzyk DW, Ruda R, Smitherman HC. Diskitis in children. Clin Orthop Relat Res. 1991:70–9. [ Links ]
19. Spiegel PG, Kengla KW, Isaacson AS, Wilson JC. Intervertebral disc-space Inflammation in Children. J Bone Joint Surg Am. 1972; 54:284-96. [ Links ]
20. Avanzi O, Chih LY, Meves R, Mattos C. Treatment of discitis in the child. Rev Assoc Med Bras. 2005; 51:113–6. [ Links ]
21. Waizy H, Heckel M, Seller K, Schroten H, Wild A. Remodeling of the spine in spondylodiscitis of children at the age of 3 years or younger. Arch Orthop Trauma Surg. 2007; 127:403-7. [ Links ]
22. Chandrasenan J, Klezl Z, Bommireddy R, Calthorpe D. Spondylodiscitis in children: a retrospective series. J Bone Joint Surg Br. 2011; 93:1122–5. [ Links ]
23. Ceroni D, Belaieff W, Kanavaki A, et al. Possible association of Kingella kingae with infantile spondylodiscitis. Pediatr. Infect. Dis. J. 2013; 32:1296–8. [ Links ]
24. Cottle L, Riordan T. Infectious spondylodiscitis. J. Infect. 2008; 56:401-12. [ Links ]
25. Colmenero JD, Jiménez-Mejías ME, Sánchez-Lora FJ, et al. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997; 56:709-15. [ Links ]
26. Kim CJ, Song KH, Jeon JH, et al. A comparative estudy of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 1976). 2010; 35:E1096-100. [ Links ]
27. Sobottke R, Seifert H, Fätkenheuer G, Schmidt M, Gossmann A, Eysel P. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008; 105:181-7. [ Links ]
28. Faella FS, Rossi M, Pagliano P, et al. Non post-operative spondylodiskitis. Our experience during the period 1990-2001. Infez Med 2002; 10:157-62. [ Links ]
29. Rudert M, Tillman B. Lymph and blood supply of the human intervertebral disc. Cadaver study of correlations to discitis. Acta Orthop Scand. 1993; 64:37-40. [ Links ]
Joana Ferreira
Department of Pediatrics
Hospital Senhora da Oliveira
Rua dos Cutileiros 114,
Creixomil, 4835-025 Guimarães
Email: joanaferreira.med@gmail.com
Received for publication: 29.12.2016
Accepted in revised form: 27.11.2017














