Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Nascer e Crescer
versión impresa ISSN 0872-0754versión On-line ISSN 2183-9417
Nascer e Crescer vol.28 no.3 Porto set. 2019
https://doi.org/10.25753/BirthGrowthMJ.v28.i3.15431
REVIEW ARTICLES | ARTIGOS DE REVISÃO
Treatment of acute migraine and status migrainosus in pediatrics
Tratamento agudo da enxaqueca e do estado de mal de enxaqueca em pediatria
Raquel Azevedo AlvesI, Marta LopesII, Ruben RochaIII, Inês CarrilhoIII
I - Department of Pediatrics, Hospital Pedro Hispano, Unidade Local de Saúde de Matosinhos. 4464-513 Senhora da Hora, Portugal. raquelazevedoalves@gmail.com
II - Department of Neurology, Hospital Senhora da Oliveira-Guimarães. 4835-044 Guimarães, Portugal marta.fmup@gmail.com
III - Department of Neuropediatrics, Centro Materno Infantil do Norte, Centro Hospitalar Universitário do Porto. 4099-001 Porto rubenrocha@gmail.com; icccarrilho@gmail.com
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Migraine is the most common acute and recurrent headache syndrome in children and adolescents but is often underdiagnosed. Migraine diagnosis in childhood rests on criteria similar to those used in adults but with some particularities, as duration of the attack, which is often much shorter than in adults, and location of the attack, which in many children may be bilateral. Despite its high prevalence, pediatric migraine remains undertreated, sometimes due to fear of caregivers and physicians and lack of studies about its treatment. Although treatment options for pediatric migraine are increasing, they remain limited.
In this article, the authors review approved and “off-label” drugs currently used in migraine and status migrainosus acute treatment in pediatric patients. In migraine treatment, nonsteroidal anti-inflammatory drugs (NSAIDs) should be used. In moderate-to-severe migraine unresponsive to analgesics or NSAIDs, triptans may be used, alone or in combination with the former. Rescue medication, including dihydroergotamine and sodium valproate, can be used in hospital setting for intractable migraine. Antiemetics with anti-dopaminergic properties may be helpful in patients with symptoms of nausea and vomiting in addition to headache, particularly when used in combination therapy.
Keywords: acute treatment; migraine; pediatric; status migrainosus
RESUMO
A enxaqueca é a cefaleia aguda e recorrente mais comum em crianças e adolescentes, mantendo-se, no entanto, subdiagnosticada. O diagnóstico de enxaqueca na infância baseia-se em critérios semelhantes aos utilizados em adultos, mas com algumas particularidades, tais como a duração da cefaleia, que geralmente é muito menor do que no adulto, e a sua localização, que pode muitas vezes ser bilateral. Apesar da elevada prevalência, a enxaqueca pediátrica continua a ser subtratada, por vezes devido a receio dos cuidadores e médicos e à falta de estudos sobre o seu tratamento. Embora as opções de tratamento para a enxaqueca pediátrica tenham vindo a aumentar, permanecem limitadas.
Neste artigo, os autores apresentam uma revisão dos fármacos atualmente aprovados e utilizados “off-label” para o tratamento agudo da enxaqueca e do estado de mal de enxaqueca em pediatria. No tratamento da enxaqueca devem ser usados anti-inflamatórios não-esteroides (AINEs). Na enxaqueca moderada a grave que não responde a analgésicos ou AINEs, podem ser utilizados triptanos, isoladamente ou em combinação com aqueles. Medicamentos de resgate, incluindo dihidroergotamina e valproato de sódio, podem ser usados em contexto hospitalar no estado de mal de enxaqueca. Antieméticos com propriedades anti-dopaminérgicas podem ser úteis em doentes com náuseas e vómitos, além de cefaleia, especialmente quando usados em combinação com outros fármacos.
Palavras-chave: tratamento agudo; enxaqueca; pediatria; estado de mal de enxaqueca
Introduction
Migraine is the most common episodic primary headache in children, with a prevalence of 1.2−3.2% at 3−7 years, 4−11% at 7−11 years, and 8−23% by the age of 15.1,2 In adults, migraine is defined by the International Classification of Headache Disorders, 3rd edition (ICHD-3) as an idiopathic, “recurring headache disorder manifesting in attacks lasting 4-72 hours. Typical characteristics of migraine are unilateral location, pulsating quality, moderate to severe intensity, aggravation by routine physical activity, and nausea and/or vomiting, or photophobia and phonophobia”.3 However, migraine diagnosis in children may be difficult.4 Children may not be able to adequately express headache characteristics. Children also tend to experience shorter attacks, lasting from two to 72 hours (h).3 Location is more likely to be bilateral, often described as frontal or bitemporal.3,4 The unilaterality, more commonly seen in adult patients, may emerge in adolescence.3 Gastrointestinal complaints, such as abdominal pain, nausea, and vomiting, are more prominent in children, and may be the sole symptoms, similarly to episodic syndromes that may be associated with migraine.4
According to results of the Global Burden of Disease 2016 report, migraine is the 6th condition with higher prevalence and the 2nd cause of Years Lived with Disability across the world.5 As such, migraine can have a substantial impact on functional ability and quality of life in pediatric patients and should be adequately treated.6-9 However, lack of evidence and insufficient clinical trials focusing pediatric migraine have been a barrier to adequate disease management.6
Migraine can be unresponsive to first-line treatments at home or in the Emergency Department (ED), and intractable cases may require hospital admission for management of pain and other associated symptoms. Admission rates for pediatric migraine patients are reported to range from 3% to as high as 32%.8 A debilitating migraine attack lasting for more than 72h is defined as status migrainosus (SM) by ICHD-3 criteria and considered a migraine complication requiring aggressive therapy for rapid relief.3 SM epidemiology in adults and children remains unknown and the condition is frequently underdiagnosed.10
The purpose of this article is to review the acute treatment of pediatric migraine and SM.
Acute treatment
The goal of migraine acute treatment is total symptom remission with minimum side effects and a rapid return to normal function. Therapy should be properly dosed, used as quickly as possible, while minimizing the potential for medication overuse. Efforts should be put into educating child as well as parents and caregivers. The child should be able to receive acute treatment as quickly as possible, at home and school, in order to minimize school absenteeism and the condition’s negative impact on social activities. Patients should to be educated about which rescue medications to use and when to use them and about avoiding medication overuse.
To avoid medication overuse headache (MOH), abortive medications should be used no more than 10 or 15 days/month, depending on the medication. Migraine-specific drugs, particularly triptans, should be used less than six times per month.4,6 Medication overuse can be a contributing factor for headache chronicity in 20-30% of children and adolescents with chronic daily headache.4
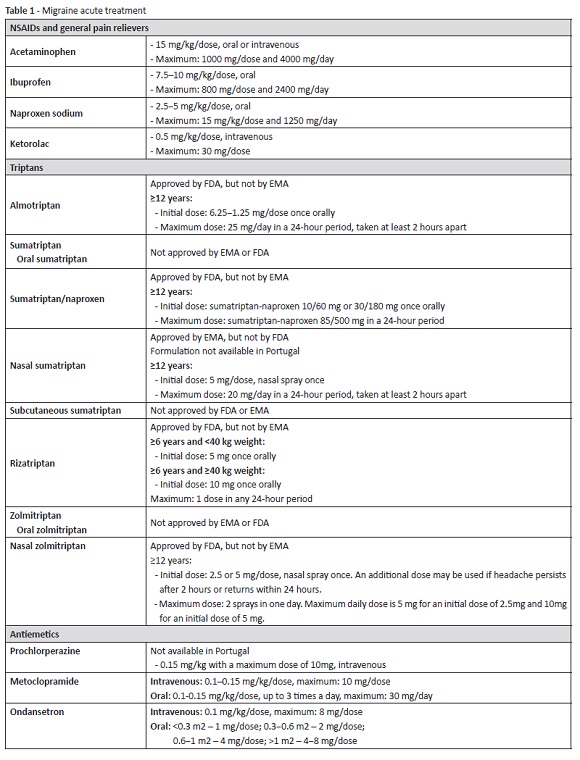
The most recent practice guidelines on the treatment of pediatric headache recommend either analgesics and/or triptans as first-line treatments for acute migraine (Table 1). In common practice, nonsteroidal anti-inflammatory drugs (NSAIDs) are used to treat mild-to-moderate migraine, with triptans reserved for moderate-to-severe cases unresponsive to analgesics.4,6
Non-pharmacological treatment
When a migraine episode starts, the child should rest or sleep in a dark and quite room. In ED setting, patients should also be placed under the same conditions whenever possible and sleep should be encouraged.1 Although non-pharmacological treatment is deemed important and can be effective per se, concomitant pharmacological treatment is frequently required and should be recommended.
Whenever possible, triggers (skipping meals, dehydration, stress, change in sleep patterns, infections, etc.) should be identified and corrected.
Pharmacological treatment
Acute management in outpatient setting
NSAIDs and general pain relievers
Few and small randomized controlled trials (RCTs) are available regarding acute treatment of migraine in children and adolescents with acetaminophen (paracetamol, as commonly designated in Europe) and ibuprofen, therefore the quality of the evidence supporting such treatments is low.11 Nevertheless, both drugs are largely used in clinical practice, with good results.
Acetaminophen/paracetamol (15 mg/kg/dose) has been shown to be safe and well tolerated, and may be considered in the treatment of children and adolescents with acute migraine.4,11-15 Due to its minimal anti-inflammatory effects, ibuprofen has been considered superior to acetaminophen.12,13,16 Acute liver failure is a serious adverse effect (AE) associated with acetaminophen and occurs mostly in cases of overdose. From the age of 12, the maximum dosing should not exceed 75 mg/kg in 24h. For children with 12 years and older, Food and Drug Administration (FDA) considers four grams the maximum safe daily dose.4,11,14 Caution should be exercised in cases of children suffering from liver damage, taking enzyme-inducing drugs, or malnourished.11
Ibuprofen at doses of 7.5-10.0 mg/kg/dose (maximum: 800 mg/ dose or 2400 mg/day) has been shown to be both safe and effective and should be considered upfront for acute migraine children without contraindications.1,4,6,12,13,15,17 Acetaminophen can be an effective alternative for children with contraindications or intolerant to NSAIDs.1,6,12,18 Naproxen sodium (2.5-5 mg/kg/dose) can be taken up to three times per day. Most common AEs associated with NSAIDs include hypersensitivity reactions, dyspepsia, and gastric bleeding. Caution should be used in patients with renal or hepatic impairment.6
The initial analgesic dose may be repeated once in every 3-4h for the same headache episode, if necessary. The use of simple analgesics should not exceed 15 days/month to prevent development of MOH.4
Other NSAIDs have not been adequately studied in pediatric migraine. Acetylsalicylic acid was investigated in adult migraine and shown to be safe and effective in selected patients. However, acetylsalicylic acid-containing products should be avoided in children under the age of 16 due to risk of Reye syndrome.4,11
As in adults combining a NSAID with a triptan has greater efficacy than either agent alone, the safety of this combination was also studied in adolescents.1,19
Triptans
Studies show that triptans are safe and effective in children and adolescents.4,11,16 Seven triptans are currently available in various formulations.4,14 However, most studies have been limited by a large placebo effect and few triptans are approved for use in children and adolescents, given the lack of RCTs on these populations.
For maximum efficacy, triptans should be taken at headache onset, and dose can be repeated once in every 2h or more if headache persists.4,8 However, no more than two triptan doses should be used in a 24-hour period.14 Patients should also be advised that triptans are not to be used within the 24-hour period of use of another triptan or with ergot-containing medications.4
Triptans should be used with extreme caution in migraine with brainstem aura and in hemiplegic migraine due to theoretical concerns of aggravating symptoms potentially elicited by vasospasm.20,21 These agents are contraindicated in patients with cerebrovascular or peripheral vascular syndromes, severe hepatic impairment, uncontrolled hypertension, hemiplegic migraine, taking monoamine-oxidase inhibitors, and during pregnancy.4
The most common triptan AEs include nausea, dizziness, fatigue, and somnolence and, in nasal formulations, taste disturbance.6,14,22-27
Almotriptan: Became the first FDA-approved triptan for treatment of acute adolescent migraine in 2009, but its use in pediatric age is not approved by the European Medicines Agency (EMA).6,22,28
Oral sumatriptan: Was not superior to placebo in available pediatric studies.6,29-32 Sumatriptan plus naproxen: Was superior to placebo for treatment of moderate-to-severe acute migraine headache in a RCT with 490 adolescents (12−17 years old) and is approved by the FDA for use in adolescents aged ≥12 years.16,19,33 It is currently not approved by EMA. Nasal sumatriptan: Is safe and effective in children with moderate-to-severe migraine over the age of eight and was the first triptan to be approved in adolescents aged ≥12 years in Europe, in 2003.6,15,16,23,24,34,35 Sumatriptan nasal spray (NS) is not only effective in relieving pain, but also in reducing photophobia and phonophobia, with rare cases of headache recurrence after initial response.24,34,35 The intranasal formulation offers many advantages, as it is rapidly absorbed, can be used in nausea and vomiting cases, and also in cases of difficult tablet swallowing.24 Sumatriptan NS is prescribed to children at the initial dose of 5 mg; if this dose is ineffective, a 10 mg dose can be attempted. In adolescents, a dose of up to 20 mg (adult dose) can be safely administered.23,24 Sumatriptan NS is well tolerated, with no serious AEs reported (currently not available in Portugal).14,23,24 Subcutaneous sumatriptan: Insufficient evidence is available to support its use in children.6,31,32,36,37 Two small open-label studies suggest that subcutaneous sumatriptan may be an effective migraine therapy.36,37
Rizatriptan: Has been shown to be an effective and well-tolerated treatment for migraine in children aged six years and over, using 5 mg for children weighing <40 kg and 10 mg for those weighing ≥40 kg.25,38,39 It is approved by the FDA for patients with 6−17 years of age, but not yet approved by EMA.6,25,32
Zolmitriptan NS: Is approved by EMA and FDA for acute migraine in pediatric patients aged 12 years and older.16,26,27 The recommended starting dose is 2.5 mg and the maximum recommended single dose is 5 mg. If necessary, a second dose can be taken after 2h, but should not exceed 10 mg in a 24-hour period. The efficacy and safety of zolmitriptan NS has not been established in pediatric patients younger than 12 years.26 Oral zolmitriptan: Three efficacy studies using different models have been conducted with oral zolmitriptan for migraine relief in children from the age of 12. Although all three studies demonstrated that oral zolmitriptan is safe, efficacy results were disparate.40-42
To date, there is insufficient data to recommend frovatriptan, naratriptan, or eletriptan in the treatment of pediatric patients with migraine.4,6,29,43
Acute management in the Emergency Department
Exhaustion of home treatment options may lead the patient and family to the ED. In ED, patient and family should be initially inquired about any previously received therapy and respective dose. Frequently, children arrive at the ED due to an initial episode or to parental fear of administering treatment. If the child has not received previous medications, treatment can be initiated with NSAIDs, oral or intravenous (IV) depending on oral tolerance.
Specific treatments available for migraine in ED setting are discussed below.
Fluids
Efficacy of IV rehydration as monotherapy in pediatric migraine treatment was assessed is a single-blind RCT conducted in patients aged between five and 17 years old.44 Patients were randomized to group A (no expectation of medication combined with IV fluid) or group B (expectation of medication given simultaneously), with both groups receiving a 10 mL/kg IV normal saline bolus. No statistical difference was found between groups. Among participants, 17.8% did however experience a clinically significant minor improvement. These findings suggest that fluids do have a modest beneficial effect in some patients, potentially those with associated nausea and emesis, more susceptible to dehydration effects.44,45
NSAIDs
Ketorolac is often given intravenously in monotherapy or combination with other drugs, mostly antidopaminergics. However, its efficacy in the pediatric patients is not as properly studied as in adults. One of the most relevant studies in the pediatric population was published in 2004 and compared parental ketorolac (0.5 mg/ kg, maximum 30 mg) with prochlorperazine in a double-blinded RCT conducted in ED setting.14,17 The study reported clinical improvement within 1h for 55.2% of children in ketorolac arm versus 84.8% of children in prochlorperazine arm and a 24-hour recurrence rate of 30% in ketorolac group.17 A possible explanation for such a high recurrence rate is the use of ketorolac in patients with analgesic rebound headache.
Dopamine receptor antagonists
Dopamine receptor antagonists (DRAs) are frequently used in acute migraine management, with some central effect on migraine symptoms and beneficial antiemetic effects, and are the mainstay of adult migraine treatment in ED setting. Studies evaluating safety and efficacy of DRAs in the pediatric population are scarce. Among investigated DRAs, IV prochlorperazine has shown the highest effectiveness.1,46-48 However, prochlorperazine is not approved by EMA for use in pediatric age, and is not available in Portugal.
Although less effective than prochlorperazine, IV metoclopramide (0.1-0.15 mg/kg, maximum 10 mg/dose) is widely used in clinical practice and has been shown to be more effective compared to placebo in pediatric migraine treatment.4,14,49 It is usually well tolerated, but extrapyramidal reactions may occur, such as dystonia and akathisia.4,14 Reducing metoclopramide infusion rate for over 15 minutes decreases sedation and akathisia, and does not impact benefits on headache and nausea.1,8
Chlorpromazine and promethazine were significantly associated with higher odds of treatment failure and higher rates of adverse events.46,50
DRAs were not shown to be more effective than ondansetron in a retrospective study, despite having been administered more than twice as often.48 Ondansetron can also be used in refractory vomiting cases.
Opioids
The American Headache Society recommends against the use of opioids for treatment of pediatric migraine, particularly in adolescents, as they may turn an episodic migraine into a more debilitating chronic headache, decrease efficacy of other medications as triptans, and increase sensitivity of pain receptors, making headaches more difficult to treat.51
Status migrainosus treatment
SM treatment is not consensual and, given its rarity, there are no guidelines in the pediatric population, with most therapeutic interventions inferred from adult treatment. Multiple therapeutic approaches are available, including intravenous hydration, parenteral DRAs, sodium valproate (VPA), dihydroergotamine, magnesium sulfate, steroids, and propofol. These are not FDA-approved for treatment of acute exacerbation of pediatric migraine, but their efficacy and tolerability have been reviewed in small pediatric studies.
Dihydroergotamine
IV dihydroergotamine (DHE) is effective in pediatric SM.52,53 Raskin protocol is the most widely accepted and has been frequently used in adults.54 Based on this protocol, two separate protocols (a high- [0.5−1 mg/dose every 8h] and a low-dose protocol) were developed is pediatrics for patients who are sensitive or unable to tolerate the higher adult dose (0.1−0.2 mg/kg every 6h).11,52,53 Response is often observed by the fifth dose and usually reaches its maximum effects by the tenth dose.51,53 If improvement is noticed, DHE should be continued until the patient is headache-free or until a maximum of 20 doses has been reached.4,11,53 Patients are typically medicated with an antiemetic 30 minutes prior to each DHE administration.16,34,55 IV DHE is not available in Europe, only oral formulations.
There is currently insufficient evidence on the use of oral DHE for treatment of children or adolescent migraine.4,55 It is not contemplated in guidelines for treatment of pediatric migraine and no oral solutions are presently available in Portugal.
Sodium valproate
VPA has been administered to children as an abortive treatment for intractable migraine and SM, with promising results.
In a retrospective review of migraine treatment with VPA in adolescents, most of which received a single 1000 mg IV VPA dose, 80% reported pain reduction, 46.8% of which with a significant headache improvement.56 In a 2015 retrospective case series, patients received a single 500 or 1000 mg IV VPA dose in ED setting. Results showed a 17% mean pain score reduction before IV VPA and an additional 40% mean reduction after VPA administration, with responses observed in a relatively short period of time.57
In 2018, Zafar et al. reported that continuous VPA infusion was effective and safe in abortive pediatric SM treatment.58 Patients received an IV VPA bolus over 30 to 60 minutes (20 mg/kg, maximum 2000 mg) followed by continuous infusion at 1 mg/kg/h. A VPA serum concentration was achieved four hours after loading dose and again 24h after continuous infusion start. Infusion rate was then adjusted to achieve 80−100 μg/ml target concentration at 24h. Once complete pain resolution or a constant pain level tolerable by the patient was achieved, IV was switched to oral extended-release formulation at the same dose the patient received in the previous 24h. Oral VPA was then weaned off, maintained at a lower prophylactic dose, or switched to another prophylactic medication. In this study, 66.2% of patients reported an excellent response, 4.8% moderate response, and 28.9% poor response.58
In all studies, VPA was generally well tolerated. IV VPA is particularly useful in basilar and hemiplegic migraine, in which IV DHE is contraindicated.59 It is also useful in patients who received ergot derivatives or triptans in the preceding hours.4
Steroids
Efficacy of steroids in SM treatment is theoretically supported by the pathophysiology of migraine attack as an inflammatory process, and these agents may be used as adjuncts to previous treatments.10 However, data is conflicting. One study suggested that steroids may decrease SM recurrence rate in the first 24−72h in adults.60 Akhtar et al. recommend using 1 mg/kg/dose methylprednisolone or 0.25−0.5 mg/kg/dose dexamethasone as loading dose in pediatric SM treatment after three patients receiving steroids showed improvement.61 On the other hand, Legault et al. found no differences between children treated and not treated with steroids.60
Magnesium sulfate
Magnesium can have a role in both neuronal and vascular theories of migraine pathogenesis and a possible association exists between intracellular magnesium concentrations and migraine attacks. In adults, evidence regarding IV magnesium efficacy is conflicting.62 In one retrospective pediatric review study, authors investigated 20 patients aged 13 to 18 years who received an IV magnesium sulfate (30 mg/kg, maximum 2000 mg) bolus infused over 30 minutes, which could be repeated in two hours if serum magnesium levels remained below an acceptable level. Seven patients (35%) showed a favorable response, five of which had SM.62
Propofol
A limited number of adult reports have shown a dramatic decrease in pain severity within 20 to 30 minutes of propofol administration for treatment of refractory headaches (“off-label” use). In these studies, propofol was administered at a subanesthetic dose not expected to produce respiratory depression or hypotension. At subanesthetic dosing, propofol has a favorable safety profile and rapid onset and offset of action, while producing hypnotic and antiemetic effects, making it ideal for potential abortive headache treatment in ED.63
Sheridan et al. compared seven pediatric patients receiving propofol with seven matched controls receiving standard institutional acute migraine therapy with NSAIDs, prochlorperazine, and diphenydramine.63 Investigators used approximately 0.56 mg/kg per bolus divided over an average of three boluses, with a bolus dose ranging from 10−50 mg (subanesthetic dosing). Patients receiving propofol achieved significantly greater pain score reductions (80.1 vs 61.1%; p<0.05) compared with matched controls. Their ED stay was shorter (122 vs 203 minutes; p<0.2), but this difference was not statistically significant. No AEs were reported.63
Propofol is not usually used in pediatrics, including in intensive care departments, due to fear of propofol-related infusion syndrome. Despite a 1.1 to 4.1% prevalence and over 64% mortality rate, this syndrome is a dose-dependent phenomenon, and preventive measures recommend avoiding propofol infusions at doses higher than 4mg/kg/h for more than 48 hours.64 However, there is insufficient data to recommend propofol in pediatric SM treatment at present.
Conclusion
Migraine is a common disorder and remains undertreated in the pediatric population. Physicians should be acquainted with the use of effective therapies capable of reducing the negative migraine impact on social and personal functioning. Based on available evidence, ibuprofen, prochlorperazine, and certain triptans are the most effective and safe agents for acute management of pediatric migraine. However, treatment of pediatric migraine is largely based on data extrapolated from adult studies, limited pediatric observational studies , and expert consensus. Further evidence-based studies are required focusing both safety and efficacy of migraine-specific agents already proven effective and currently used in adult migraine treatment. Such information could then be incorporated into new practice guidelines on the treatment of pediatric migraine, with subsequent standardization of care.
REFERENCES
1. Gelfand AA, Goadsby PJ. Treatment of Pediatric Migraine in the Emergency Room. Pediatr Neurol. 2012; 47:233-41. [ Links ]
2. Lewis DW. Pediatric Migraine. Neurol Clin. 2009; 27:481-501. [ Links ]
3. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; 38:1-211. [ Links ]
4. Kacperski J, Kabbouche MA, O’Brien HL, Weberding JL. The optimal management of headaches in children and adolescents. Ther Adv Neurol Disord. 2016; 9:53-68.
5. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390:1211-59. [ Links ]
6. O’Brien HL, Kabbouche MA, Hershey AD. Treating pediatric migraine: an expert opinion. Expert Opin Pharmacother. 2012; 13:959-66.
7. Powers SW, Patton SR, Hommel KA, Hershey AD. Quality of Life in Childhood Migraines: Clinical Impact and Comparison to Other Chronic Illnesses. Pediatrics. 2003; 112:e1-5. [ Links ]
8. Kaar CRJ, Gerard JM, Nakanishi AK. The Use of a Pediatric Migraine Practice Guideline in an Emergency Department Setting. Pediatr Emerg Care. 2015; 32:435-9. [ Links ]
9. Kernick D, Reinhold D, Campbell JL. Impact of headache on young people in a school population. Br J Gen Pract. 2009; 59:678-81. [ Links ]
10. Kabbouche M. Pediatric Inpatient Headache Therapy: What is Available. Headache 2015; 55:1426-9. [ Links ]
11. O’Brien HL, Kabbouche MA, Kacperski J, Hershey AD. Treatment of Pediatric Migraine. Curr Treat Options Neurol. 2015; 17:326-43.
12. Hämäläinen ML, Hoppu K, Valkeila E, Santavuori P. Ibuprofen or acetaminophen for the acute treatment of migraine in children: a double-blind, randomized, placebo-controlled, crossover study. Neurology. 1997; 48:103-7. [ Links ]
13. Freitag FG, Schloemer F, Shumate D. Recent developments in the treatment of migraine in children and adolescents. Headache Pain Manag. 2016; 1:1-8. [ Links ]
14. Sheridan DC, Spiro DM, Meckler GD. Pediatric Migraine: Abortive Management in the Emergency Department. Headache 2014; 54:235-45. [ Links ]
15. Lewis D, Ashwal S, Hershey A, Hirtz D, Yonker M, Silberstein S. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology 2004; 63:2215-24. [ Links ]
16. Richer L, Billinghurst L, Linsdell MA, Russel K, Vandermeer B, Crumley ET, et al. Drugs for the acute treatment of migraine in children and adolescents. Cochrane Database of Systematic Reviews 2016; 4:CD005220. [ Links ]
17. Brousseau DC, Duffy SJ, Anderson AC, Linakis JG. Treatment of pediatric migraine headaches: a randomized, double-blind trial of prochlorperazine versus ketorolac. Ann Emerg Med. 2004; 43:256-62. [ Links ]
18. Brenner M, Lewis D. The treatment of migraine headaches in children and adolescents. J Pediatr Pharmacol Ther. 2008; 13:17-24. [ Links ]
19. McDonald SA, Hershey AD, Pearlman E, Lewis D, Winner PK, Rothner D, et al. Long-Term Evaluation of Sumatriptan and Naproxen Sodium for the Acute Treatment of Migraine in Adolescents. Headache. 2011; 51:1374-87. [ Links ]
20. Klapper J, Mathew N, Nett R. Triptans in the treatment of basilar migraine and migraine with prolonged aura. Headache. 2001; 41:981-4. [ Links ]
21. Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol. 2011; 10:457-70. [ Links ]
22. Linder SL, Mathew NT, Cady RK, Finlayson G, Ishkanian G, Lewis DW. Efficacy and Tolerability of Almotriptan in Adolescents: A Randomized, Double-Blind, Placebo-Controlled Trial. Headache. 2008; 48:1326-36. [ Links ]
23. Winner P, Rothner AD, Saper J, Nett R, Asgharnejad M, Laurenza A, et al. A Randomized, Double-Blind, Placebo-Controlled Study of Sumatriptan Nasal Spray in the Treatment of Acute Migraine in Adolescents. Pediatrics. 2000; 106:989-97. [ Links ]
24. Ahonen K, Hämäläinen ML, Rantala H, Hoppu K. Nasal sumatriptan is effective in treatment of migraine attacks in children: A randomized trial. Neurology. 2004; 62:883-7. [ Links ]
25. Ahonen K, Hämäläinen ML, Eerola M, Hoppu K. A randomized trial of rizatriptan in migraine attacks in children. Neurology. 2006; 67:1135-40. [ Links ]
26. McKeage K. Zolmitriptan Nasal Spray: A Review in Acute Migraine in Pediatric Patients 12 Years of Age or Older. Paediatr Drugs. 2016; 18:75-81. [ Links ]
27. Winner P, Farkas V, Stillová H, Woodruff B, Liss C, Lillieborg S, et al. Efficacy and tolerability of zolmitriptan nasal spray for the treatment of acute migraine in adolescents: Results of a randomized, double-blind, multi-center, parallel-group study (TEENZ). Headache. 2016; 56:1107-19. [ Links ]
28. Charles JA. Almotriptan in the acute treatment of migraine in patients 11-17 years old: an open-label pilot study of efficacy and safety. J Headache Pain. 2006; 7:95-7. [ Links ]
29. Eiland LS, Hunt MO. The use of triptans for pediatric migraines. Paediatr Drugs. 2010; 12:379-89. [ Links ]
30. Fujita M, Sato K, Nishioka H, Sakai F. Oral sumatriptan for migraine in children and adolescents: a randomized, multicenter, placebo-controlled, parallel group study. Cephalalgia. 2014; 34:365-75. [ Links ]
31. Hämäläinen ML, Hoppu K, Santavuori P. Sumatriptan for migraine attacks in children: a randomized placebo-controlled study. Do children with migraine respond to oral sumatriptan differently from adults? Neurology. 1997; 48:1100-3. [ Links ]
32. Sakai F. Oral Triptans in Children and Adolescents: An Update. Curr Pain Headache Rep. 2015; 19:342-4. [ Links ]
33. Derosier FJ, Lewis D, Hershey AD, Winner PK, Pearlman E, Rothner AD, et al. Randomized trial of sumatriptan and naproxen sodium combination in adolescent migraine. Pediatrics. 2012; 129:e1411-20. [ Links ]
34. Damen L, Brujin JK, Verhagen AP, Berger MY, Passchien J, Koes BW. Symptomatic treatment of migraine in children: a systematic review of medication trials. Pediatrics. 2005; 116:e295-302. [ Links ]
35. Ueberall MA, Wenzel D. Intranasal sumatriptan for the acute treatment of migraine in children. Neurology. 1999; 52:1507- 10. [ Links ]
36. Linder SL. Subcutaneous sumatriptan in the clinical setting: the first 50 consecutive patients with acute migraine in a pediatric neurology office practice. Headache. 1996; 36:419-22. [ Links ]
37. MacDonald JT. Treatment of juvenile migraine with subcutaneous sumatriptan. Headache. 1994; 34:581-2. [ Links ]
38. Ho TW, Pearlman E, Lewis D, Hämäläinen ML, Connor K, Michelson D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: Results from a randomized, double-blind, placebo-controlled trial using a novel adaptive enrichment design. Cephalalgia. 2012; 32:750-65. [ Links ]
39. Hewitt DJ, Pearlman E, Hämäläinen ML, Lewis D, Connor KM, Michelson D, et al. Long-Term Open-Label Safety Study of Rizatriptan Acute Treatment in Pediatric Migraineurs. Headache. 2012; 53:104-17. [ Links ]
40. Linder SL, Dowson AJ. Zolmitriptan provides effective migraine relief in adolescents. Int J Clin Pract. 2000; 54:466-9. [ Links ]
41. Rothner AD, Wasiewski W, Winner P, Lewis D, Stankowski J. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache. 2006; 46:101-9 . [ Links ]
42. Evers S. Rahmann A, Kraemer C, Kurlemann G, Debus O, Husstedt IW, et al. Treatment of childhood migraine attacks with oral zolmitriptan and ibuprofen. Neurology. 2006; 67:497- 9. [ Links ]
43. Winner P, Linder SL, Lipton RB, Almas M, Parsons B, Pitman V. Eletriptan for the Acute Treatment of Migraine in Adolescents: Results of a Double-Blind, Placebo-Controlled Trial. Headache. 2007; 47:511-18. [ Links ]
44. Richer L, Craig W, Rowe B. Randomized Controlled Trial of Treatment Expectation and Intravenous Fluid in Pediatric Migraine. Headache. 2014; 54:1496-505. [ Links ]
45. Patniyot IR, Gelfand AA. Acute Treatment Therapies for Pediatric Migraine: A Qualitative Systematic Review. Headache. 2016; 56:49-70. [ Links ]
46. Sheridan DC, Laurie A, Pacheco S, Fu R, Hansen ML, Ma OJ, et al. Relative Effectiveness of Dopamine Antagonists for Pediatric Migraine in the Emergency Department. Pediatr Emerg Care. 2018; 34:165-8. [ Links ]
47. Kabbouche MA, Vockell AL, LeCates SL, Powers SW, Hershey AD. Tolerability and Effectiveness of Prochlorperazine for Intractable Migraine in Children. Pediatrics. 2001; 107:e62-4. [ Links ]
48. Bachur RG, Monuteaux MC, Neuman MI. A Comparison of Acute Treatment Regimens for Migraine in the Emergency Department. Pediatrics. 2015; 135:232-8. [ Links ]
49. Coppola M, Yealy DM, Leibold RA. Randomized, placebo-controlled evaluation of prochlorperazine versus metoclopramide for emergency department treatment of migraine headache. Ann Emerg Med. 1995; 26:541-6. [ Links ]
50. Kanis JM, Timm NL. Chlorpromazine for the treatment of migraine in a pediatric emergency department. Headache. 2014; 54:335-42. [ Links ]
51. Sheridan DC, Meckler GD. Inpatient Pediatric Migraine Treatment: Does Choice of Abortive Therapy Affect Length of Stay? J. Pediatr. 2016; 179:211-5. [ Links ]
52. Linder SL. Treatment of Childhood Headache with Dihydroergotamine Mesylate. Headache. 1994; 34:578-80. [ Links ]
53. Kabbouche MA, Powers SW, Segers A, LeCates S, Manning P, Biederman S, et al. Inpatient Treatment of Status Migraine With Dihydroergotamine in Children and Adolescents. Headache. 2009; 49:106-9. [ Links ]
54. Raskin NH. Treatment of status migrainosus: the American experience. Headache. 1990; 30:550-3. [ Links ]
55. Hämäläinen ML, Hoppu K, Santavuori PR. Oral dihydroergotamine for therapy-resistant migraine attacks in children. Pediatr Neurol. 1997; 16:114-7. [ Links ]
56. Reiter PD, Nickisch J, Merritt G. Efficacy and tolerability of intravenous valproic acid in acute adolescent migraine. Headache. 2005; 45:899-903. [ Links ]
57. Sheridan D, Sun B, O’Brien P, Hansen M. Intravenous Sodium Valproate for Acute Pediatric Headache. J Emerg Med. 2015; 49:541-5.
58. Zafar MS, Stewart AM, Toupin DN, Cook AM, Baumann RJ. Continuous Intravenous Valproate as Abortive Therapy for Pediatric Status Migrainosus. Neurologist. 2018; 23:43-6. [ Links ]
59. Mathew NT, Kailasam J, Meadors L, Chernyschev O, Gentry P. Intravenous valproate sodium (depacon) aborts migraine rapidly: a preliminary report. Headache. 2000; 40:720-3. [ Links ]
60. Legault G, Eisman H, Shevell MI. Treatment of Pediatric Status Migrainosus: can we prevent the “bounce back”? J Child Neurol. 2011; 26:949-55.
61. Akhtar ND, Murray MA, Rothner AD. Status migrainosus in children and adolescents. Semin Pediatr Neurol. 2001; 8:27- 33. [ Links ]
62. Gertsch E, Loharuka S, Wolter-Warmedam K, Tong S, Kempe A, Kedia S. Intravenous Magnesium As Acute Treatment for Headaches: A Pediatric Case Series. J Emerg Med. 2014; 46:308-12. [ Links ]
63. Sheridan DC, Spiro DM, Nguyen T, Koch TK, Meckler GD. Low- Dose Propofol for the Abortive Treatment of Pediatric Migraine in the Emergency Department. Pediatr Emerg Care. 2012; 28:1293-6. [ Links ]
64. Zhidambaran V, Costandi A, D’Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2018; 32:543-63.
Endereço para correspondência | Dirección para correspondencia | Correspondence
Raquel Azevedo Alves
Department of Pediatrics
Hospital Pedro Hispano
Unidade Local de Saúde de Matosinhos.
Rua do Doutor Eduardo Torres
4464-513 Senhora da Hora
Email: raquelazevedoalves@gmail.com
Received for publication: 11.11.2018
Accepted in revised form: 01.04.2019