A 15-year-old boy presented with pain in the right ankle with one month of evolution. He had been treated for chronic myeloid leukemia (CML) with blast crisis five years before, with allogeneic bone marrow transplantation in the following year. One year after transplantation, the boy presented with CML recurrence and underwent a second transplant, with a new recurrence 12 months later.
Right ankle radiograph showed a mixed lesion with sclerotic and lytic areas in the anterior aspect of the tibia metaphysis (Figures IA and IB). Right ankle magnetic resonance imaging detected an infiltrative low-signal lesion located in the anterior aspect of the distal tibia metaphysis, with epiphyseal and extraosseous extension, associated joint effusion, and adjacent soft tissue edema (Figure IC).
Anatomopathological evaluation reported a neoplastic infiltrate composed of anomalous hematopoietic cells with immature aspect, including CD45 immunoexpression, heterogeneous CD38, and partial CD7, CD66c, CD9, and CD10 immunoexpression, permeated by a mixed inflammatory infiltrate.
What is your diagnosis?
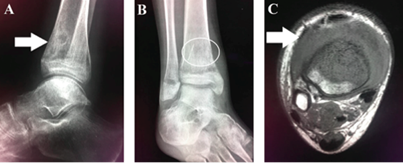
Figure IA X-ray of the right ankle on lateral view showing a mixed lesion with sclerotic and lytic areas in the anterior aspect of the distal tibia metaphysis (white arrow). Figure IB - X-ray of the right ankle on anteroposterior view showing a mixed lesion with sclerotic and lytic areas in the distal tibia metaphysis (white circle). Figure IC - MRI of the right ankle on axial T1-weighted imaging showings an infiltrative low-signal lesion located in the anterior aspect of the distal tibia metaphysis and extraosseous extension, with adjacent soft tissue edema (white arrow).
Discussion
In this clinical case, diagnostic findings, adding to the history of CML, were compatible with granulocytic sarcoma.
Granulocytic sarcoma, also known as chloroma or myeloid sarcoma, is a rare extramedullary tumor characterized by one or more tumor masses consisting of myeloblasts or immature myeloid cells that disrupt the normal architecture of involved tissues, and typically occurs concurrently with acute myeloid leukemia (AML).1-4 It was first described by Burns in 1811 as a “green tumor”, due to its greenish hue resulting from presence of myeloperoxidase.3-5
The condition can also occur in association with accelerated-phase CML or myelodysplastic syndrome,1-8 as extramedullary AML relapse (including in post-bone marrow transplant setting), and occasionally as first presenting manifestation, even before bone marrow involvement.1,2 The incidence of granulocytic sarcoma with AML is 1.4−8.0%.2,7,9 Increased incidence of granulocytic sarcoma has been reported after allogenic bone marrow transplantation.4
Depending on the specific location and size, clinical manifestations are heterogeneous, making diagnostic challenging, particularly in patients without initial bone marrow involvement.1 Very rarely, granulocytic sarcoma can occur in absence of systemic disease and predate the onset of an underlying hematologic malignancy by months to years.4,7
Granulocytic sarcoma may present at any age5 and occur in any part of the body.4,5 The most common presentation sites are the skin (28.2%), lymph nodes (16.3%), testis (6.5%), and intestine (6.5%),2,8 with central nervous system and bone accounting for a 3.25% frequency each.2,8 It occurs in the spine in 1.0% of all patients with AML or CML, more frequently in the lumbosacral, followed by thoracic and cervical spine.6,7 The skin and orbit are the most frequently affected sites in children.1
Diagnostic tools for correct diagnosis of granulocytic sarcoma are important and should include magnetic resonance imaging (MRI) and/or computed tomography (CT) scan for evaluating tumor size and location and for distinguishing the tumor from other lesions; bone marrow and peripheral blood morphological and flow cytometric analysis; and myeloid sarcoma biopsy and immunohistochemical staining in patients without bone marrow involvement.1
In the central nervous system, MRI shows lesions of variable size as isointense or hypointense compared with normal grey matter on T1- and T2-weighted images, with marked homogeneous contrast enhancement.2,3,5 Tumor signal intensity on T2‑weighted images is due to high levels of myeloperoxidase, an iron‑containing enzyme normally found in white blood cells.5 In other body sites, lesions are isointense to hypointense compared with skeletal muscle on T1-weighted images, and mildly hyperintense on T2-weighted images, usually showing homogeneous enhancement greater than the observed in muscle.3,7
When bone is affected, lesion appears lytic rather than sclerotic.2,10 Both CT scan and MRI are extremely useful for diagnosis.10 On CT scan, a well-defined area of decreased or increased attenuation with a peripheral enhancement area can be found.10
MRI reveals the exact tumor extent, as well as potential invasion to surrounding structures.2 However, without an hematological disorder, lesions are difficult to differentiate solely based on image findings from metastases or other tumors, as lymphomas or inflammatory granulomas, and dural-based lesions can mimic meningiomas.2
Spinal granulocytic sarcoma should be considered when a solitary dumbbell-shaped mass in the intervertebral foramen with diffuse bone marrow infiltration is shown on MRI.9 If this pathology is present, imaging evaluation should be extended to the head.9 Chaudhry et al. reported a diffusion restriction in 96% of patients with cerebral myeloid sarcoma on diffusion-weighted images.4,11 It should be acknowledged that, neither signal intensity on MRI and CT scan, nor contrast enhancement are specific for chloroma lesions.4
According to the literature, another beneficial imaging modality is positron emission tomography (PET)/CT, which is more accurate than CT scan alone in diagnosing chloroma.4 As reported, chloroma lesions show elevated fluorodeoxyglucose uptake, which changes under therapy and correlates with clinical outcome.4 The main differential diagnosis to consider is malignant lymphoma.5
Histologically, chloroma is composed of sheets of immature myeloid cells, mainly myeloblasts, that infiltrate the involved tissue.3,6 CD68, lysozyme, and CD43 are among the most widely expressed markers in immunohistochemical staining, with variable expression of a wide range of other antigens, including myeloperoxidase, CD33, CD34, CD117 (c-Kit), CD4, CD56, and terminal deoxynucleotidyl transferase, depending on the lineage and maturation stage.3 CD68/KP1 is the most commonly expressed marker.5
It should be emphasized that granulocytic sarcoma diagnosis is particularly challenging, even for the pathologist. As radiography and MRI are not specific to the condition, other differential diagnoses can be evoked, with clinical and personal history being determinant factors for final diagnosis.3,6
Due to its excellent soft-tissue contrast, MRI is particularly useful for treatment planning.3 Granulocytic sarcoma treatment includes AML-based protocols, surgery, and/or radiotherapy in cases of symptomatic lesions or tumors causing local organ dysfunction.1 Cytarabine is a key drug in the regimen.5 There is currently no evidence that adding radiotherapy has a positive effect on survival compared to chemotherapy alone.5 Patients with granulocytic sarcoma have poor prognosis, with a reported overall survival of 15.9 months.2,4,8














