Introduction
Hymenoptera is an insect order very common throughout Europe including, among others, wasps (family: Vespidae; genus: Dolichovespula, Polistes, Vespula) and bees (family: Apidae; genus: Apis, Bombus).1 The prevalence of hymenoptera venom allergy (HVA) in this continent is estimated to be around 20%.2
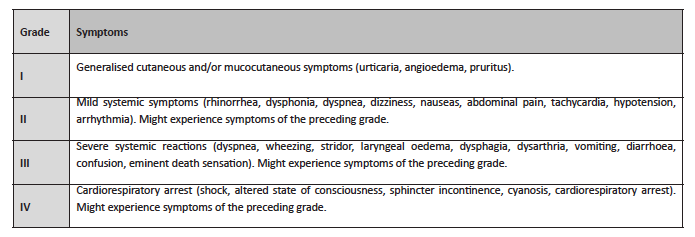
These insects produce venom with potentially allergenic elements. Bee venom includes phospholipase A2 (Api m1), hyaluronidase (Api m2), melittin (Api m3), acid phosphatase (Api m4), apamin, and peptide 401, while wasp venom’s composition includes phospholipase A1 (Ves v1), hyaluronidase (Ves v2), acid phosphatase, antigen 5, a neurotoxin (Ves v5), and quinine.3 Both can trigger grade I hypersensitivity reactions mediated by immunoglobulin E (IgE), which may manifest as grade II and III local or systemic reactions, either moderate or severe (Table 1).2
There is significant cross-reactivity between Apidae and Vespidae family venom. Between bees and wasps, it happens due to hyaluronidase homology and, although to a lesser extent, to cross-reactive carbohydrate determinants (CCDs). Around 30% of people who are allergic to venoms are positive for bee and wasp in vitro, but this rarely happens in intradermal skin test diagnosis.4,5
Measuring the species-specific major molecular allergens enables to diagnose the true cases of double sensitisation in such cross-reactivity instances, by detecting the simultaneous presence of IgE to both Api m1 and Ves v5.4,5
Molecular allergens are also useful in distinguishing between Vespula and Polistes.
Unfortunately, most patients have difficulty in identifying which insect stung them and caused the allergic reaction.1
Bees inject between 50 and 100 μg of venom per sting, but the value can amount to about 300 μg when the venom sac is emptied. The bee dies after the sting. The wasp, on the other hand, injects around 1.7 to 3 μg of venom, being able to inflict several stings without dying.6
Systemic reactions in childhood are uncommon, with an estimated prevalence between 0.15-0.8%. The prevalence is higher in adults (0.3 to 8.9%).7 The annual mortality associated with this allergy is 0.03-0.48 deaths/1.000,000 population1. About 30% of anaphylaxis cases seen in Emergency Departments are associated with hymenoptera stings.8
The diagnosis is mostly based on medical history, with identification of the culprit insect assuming a crucial importance.
Even though currently available molecular allergens improve the diagnostic acuity, skin tests remain the gold standard in HVA diagnosis, with intradermal test offering the most sensitive procedure. These skin tests must be performed at least two to six weeks after the sting to avoid possible false negatives, due to the refractory period - anergy1. In the first few days after a sting, the injected venom-specific IgE may be low or even undetectable. If negative and in presence of a definitive history of systemic sting reaction, skin test should be repeated after one to two months, considering that refractoriness duration may be longer.1
HVA treatment implies the adoption of eviction measures and administration of adrenaline, antihistamine, and oral corticosteroid according to need. The only curative treatment described is the Hymenoptera venom-specific immunotherapy (VIT).1 It is indicated in children with mild-to-severe systemic allergic reactions with affection of more than one organ. Children with extensive local reaction who live in hymenoptera endemic areas - therefore incurring in increased exposition probability - and who experience problems due to repeated reactions should be individually assessed. VIT has a 91−96% efficiency rate in cases of wasp venom allergy and 77−84% in cases of bee venom allergy.. The duration of VIT treatment remains controversial.9
Case report
An eight -year-old male patient was referred to the Allergy and Clinical Immunology (ACI) Department due to suspicion of allergy to hymenoptera venom. He denied usual medications, known medications, and/or food allergies. His father was a beekeeper.
The first sting occurred at the age of two years, in the scalp, with no local or systemic reaction. After five years, another sting in the right hand induced facial and right hemibody angioedema, dyspnea, chest discomfort with a feeling of breathness, and glottic edema - grade III systemic reaction. The boy was treated in the Emergency Department, receiving subcutaneous adrenaline injection 0.01 mg/Kg and intravenous clemastine 0.025 mg/Kg. He was discharged 12 hours later, hemodynamically stable and without symptomatology.
Skin prick tests for bee, Vespula, and Polistes venom were performed using Leti® commercial allergen extract, with results proving positive for bee venom extract (concentration of 0.01 µg/ml) and negative for Vespula and Polistes venom (tested up to the 100 µg/ml dose): negative control - saline solution 0 mm, histamine 6 mm, bee (0.01 µg/ml) 8 mm, Vespula 0 mm, Polistes spp. 0 mm. Quantification of specific IgE (kUA/L) was 41.90 for bee, 0.34 for Vespula spp., and 0.32 for Polistes spp. venoms. Intradermal tests were not performed.
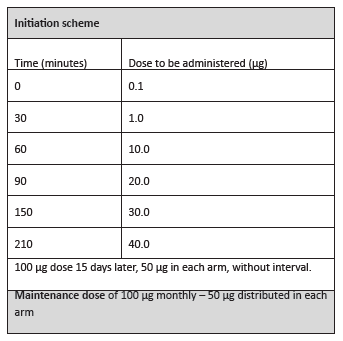
In the clinical follow-up consultation, the boy exhibited no relevant physical changes. He was medicated with auto-injectable adrenaline 0.3 mg, prednisolone 40 mg per os, and levocetirizine 5 mg for systemic reactions, and advised about eviction measures against hymenoptera. Subcutaneous VIT for Apis mellifera by Leti® laboratories was prescribed. He started treatment at the Day Hospital according to the ultra-rush scheme (Table 2).
In the first and second vaccine administrations, the patient experienced severe grade III systemic reactions (diffuse pruritic exanthema, angioedema, dry cough, dyspnea, and bronchospasm), requiring intramuscular adrenaline 0.3 mg, nebulization with salbutamol 5 mg/2.5ml, and intravenous clemastine 0.025 mg/kg/dose. Such measures resulted in resolution of clinical features and enabled to continue VIT.
Subsequent administrations were performed under pre-medication, namely prednisolone 40 mg and desloratadine 5 mg per os. However, in the seventh and twelfth administrations, recurrence of systemic reactions during VIT administration similar to those previously described prompted the implementation of crisis treatment.
After six months of treatment, the patient stopped requiring pre-medication and experienced no further (cutaneous or systemic) reactions with VIT.
In the twelfth and again in the twenty-seventh month of treatment, the patient was accidentally stung by a bee on the left arm, experiencing only a local reaction through development of a 4-mm papule, with no other related symptomatology and spontaneous resolution. At thirty-eight months of treatment, he was again stung by a bee on the right arm, without local or systemic reactions.
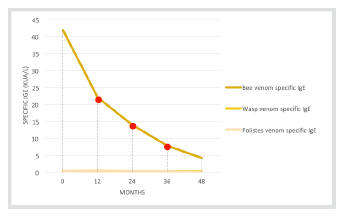
Throughout treatment, bee, Vespula, and Polistes venom-specific IgE measurements were taken. Bee venom-specific IgE showed a significant decrease over time (Figure 1).
The patient completed five years of treatment in the end of 2019. Currently, he presents no local or systemic reactions with VIT and remains clinically stable.
Discussion/conclusion
Several risk factors for severe systemic reactions have been described, including age (more relevant in the elderly); occurrence of a previous reaction and its severity; time elapsed between the two stings (the smaller it is, the higher the risk of a future severe systemic reaction); type of hymenoptera (higher risk for bee stings); elevated baseline serum tryptase; cardiovascular diseases, systemic mastocytosis, and autoimmune diseases; and use of beta-blocker drugs (reduce adrenaline efficacy during anaphylactic treatment) and angiotensin-converting-enzyme inhibitors (ACE inhibitors).1,6
VIT safety and efficacy make it adequate for pediatric ages. It can nonetheless be linked to both local (50%) and systemic (2−20%) reactions, possibly manageable with medication, as in the present case.9
The ultra-rush scheme, one of the most frequently used along with the rush scheme, was used in the present patient as a way to ensure compliance and achieve a maintenance dose as early as possible, considering that the patient lived far from the hospital. Dose-associated errors are also reduced in accelerated schemes, due to the lower number of administrations and shorter period of time.10,11 Additionally, ultra-/rush schemes are at least as safe as traditional ones.12
In this case, due to adverse reactions in the first administrations, prophylaxis was needed. Prednisolone was used - medium action glucocorticoid (12 to 36h) with anti-inflammatory activity in doses between 1 to 2 mg/Kg/day −, as well as anti-histaminic H1 - desloratadine, to prevent histamine induced effects. If reactions persist, administration of anti-IgE omalizumab before VIT should be considered. This treatment is safe from the age of six years. As an alternative, maintenance dose could be reduced in 50%. One study showed that using the 50 μg instead of the 100 μg dose in children was safe but had lower efficacy, not enough to be recommended.4 Having attained VIT maintenance dose, there is a 75−95% prevention rate of new systemic reactions after a sting, as opposed to 40−60% in non-treated patients.2
VIT mechanisms of action are not entirely clear. It is however acknowledged that the insect’s venom-specific IgE tends to increase in treated patients in the first months and decrease afterwards, with IgG4 rapidly rising in the beginning of treatment and retaining high values throughout.3 Changes in T cell cytokine profile also occur, with redirection of T helper cells type 2 (IL-4,5) to T helper type 1 (interferon-γ) and regulatory T cells (IL-10 and IgG4) response increase. There is also a decline in CD63 expression in mast cells’ membrane.
Despite representing favorable factors, specific IgEs and cutaneous reactivity may not offer an absolute correlation with therapeutic success, reason why monitoring their levels is not mandatory. Only a new sting by the culprit hymenoptera can confirm therapy effectiveness, but such a test poses several ethical issues.9 In the reported case, a significant decrease of specific IgEs was observed since the beginning of treatment, contrarily to what is described in the literature. As previously mentioned, a new bee sting caused only a local reaction and a second bee sting caused no reaction at all.
Treatment duration remains controversial. Several studies indicate that clinical protection is achieved as soon as the maintenance dose is reached, with reports describing relapse rates between 22 and 27% two years after discontinuing VIT.9 The same studies concluded that three-year-long VIT can be effective, especially in patients with only slight-to-moderate reactions.9 It has been demonstrated that 80−90% of patients become protected after five years of treatment.1 However, in increased risk cases, namely in patients with initial severe systemic reactions or adverse reactions during VIT or in patients allergic to bee venom with a higher risk of being stung again, a longer treatment period may be necessary, as in the present case.1,9
HVA, apart from interfering with patients’ quality of life, can trigger potentially fatal severe systemic reactions, as anaphylaxis. It represents a medical emergency that should be directed to an ACI Department, as VIT represents the only effective treatment. Still, a wide lack of information persists regarding this allergy, with patients belatedly pointed to the ACI Department.
Molecular allergens may have a rather important role in guiding and choosing VIT for these patients’ treatment. However, there is a clear need for more information and greater availability of these allergens.1
In this case, the use of complementary diagnostic exams, like skin prick tests, could be excluded, as the hymenoptera responsible for the reaction was previously acknowledged and the identified IgE confirmed the diagnosis. Iatrogenesis associated with these exams (pain, fear, allergic reaction) should be considered.
There is no consensus about maintaining auto-injectable adrenaline after finishing treatment. This option was discussed with the patient, who decided to keep the pen after the five-year treatment, as it reduced anxiety from a possible new anaphylactic reaction. Nevertheless, the patient has been field stung after treatment without systemic reactions.