Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent responsible for the coronavirus disease 2019 (COVID-19), emerged in China in early December 2019.1 The outbreak was declared a public health emergency of international concern by the World Health Organization (WHO) on January 30, 2020, with the first case reported in Portugal in March 2020.2,3) During these pandemic years, children infected with SARS-CoV-2 were usually asymptomatic or presented with mild coronavirus disease.4
While acute symptoms are well defined and extensively described, the long-term effects of COVID-19 are less clear due to the yet short follow-up since the outbreak. Most COVID-19 patients achieve full recovery within 3-4 weeks after the onset of the infection, but in some cases, symptoms may persist weeks or months after the recovery. In adults, one in every five patients reports symptoms beyond five weeks, and one in every ten patients presents symptoms beyond 12 weeks.5) These symptoms appear to occur irrespective of the initial severity of the infection.
Although several authors have sought to define and understand the persistency of these symptoms, consensus was not achieved. The absence of a single terminology and clinical case definition were drawbacks to moving forward on epidemiological reporting and clinical management of these patients. To standardize nomenclature, WHO proposed definitions in October 2021. Post-COVID syndrome was defined as a condition in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually three months after the onset of COVID-19, with symptoms that last for at least two months and cannot be explained by an alternative diagnosis.6) In February 2022, the National Institute for Health and Care Excellence (NICE) published guidelines to define Long COVID. According to this organization, symptoms from the onset of the disease to four weeks after diagnosis are considered to concern acute COVID-19 infection. When symptoms persist from 4 to 12 weeks, the infection is termed ongoing symptomatic COVID-19, and when symptoms persist beyond 12 weeks, it is termed post-COVID-19. Both ongoing symptomatic COVID-19 and post-COVID-19 are considered Long COVID.7) In children, the evidence regarding persistent COVID-19 symptoms remains scarce.
Objective
The aim of this study was to review the available literature regarding Long COVID syndrome in children and adolescents.
Methods
A non-systematic literature review was conducted in May, June, and July 2022 on PubMed and Google Scholar databases using the terms “Long COVID”, “post-COVID”, “persistent COVID” AND “children”, “adolescents”, “pediatric”. Initial article selection was carried out by reading their titles and abstracts and selecting those with relevant content for the review. Only English-language articles were considered. There were no restrictions on journals or publication date. All journals were indexed, with the lowest impact factor being 2.898 and the highest 157.3. Observational studies, including cohort and cross-sectional studies, were analyzed. Systematic reviews, case reports, studies with combined adult and pediatric data , and articles focusing only acute COVID-19 were excluded.
Results
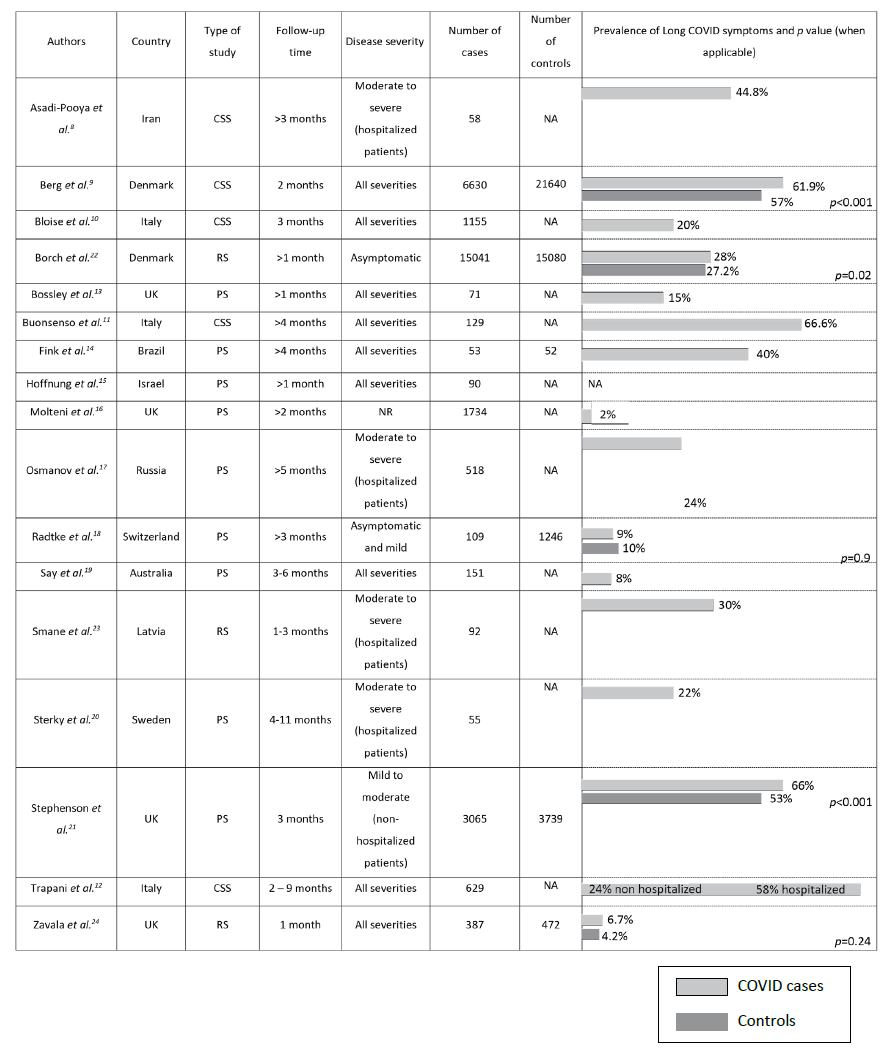
A total of 17 studies were identified in the literature based on the predefined inclusion criteria: five cross-sectional studies, nine prospective studies, and three retrospective studies (Table 1).8,12,13,22-24 The number of children and adolescents included in studies varied from 58 to 30121 (median, 518). Children were assessed for Long COVID symptoms for varying lengths of time, ranging from one to 11 months. Symptom assessment was conducted through telephone survey in six studies, electronic survey in five studies, paper survey in two studies, and clinical visits in four studies.8-24
Prevalence of long covid symptoms
The prevalence of Long COVID symptoms differed considerably among studies, ranging from 2 to 66%. Persistent symptoms and their frequency also greatly varied among studies. The most common symptoms were fatigue (2─85%), shortness of breath (5─50%), headache (3─29%), sleep disturbance (4─33%), concentration difficulties (4─21%), chronic cough (1─29%), dizziness (2─19%), myalgia (2─46%), chest pain (1─31%), poor sense of smell or anosmia (1─26%), abdominal pain (1─20%), and loss of appetite or weight (5─19%).
Risk factors for the development of long covid
Eight studies sought to identify risk factors associated with the development of Long COVID.8,10,12,15-17,21,22 All found a positive correlation with age, with older children and adolescents having an increased risk for Long Covid. Asadi-Pooya et al. showed that muscle pain on admission and Intensive Care Unit (ICU) admission were significantly associated with Long COVID.8 Osmanov et al. found a positive correlation with history of allergic diseases,17 and Bloise et al. identified higher body mass index (BMI) and longer duration of infection as risk factors.10 In addition, Trapani et al. reported that children aged 0 to 5 years had a greater risk of developing respiratory symptoms, while adolescents (aged 11-16 years) had a greater risk of neurological and psychological Long COVID-19 symptoms.12
Hospitalized vs. Non-hospitalized patients
Four of the studies retrieved included only hospitalized patients, nine included children and adolescents with all disease severities, two included only asymptomatic or mildly symptomatic patients, one included only non-hospitalized patients, and one included and compared hospitalized and non-hospitalized patients.8-15,17-20,22-24 The latter reported significant differences between groups in the incidence of Long COVID, with 58% of hospitalized patients versus 24.3% of patients in the primary care setting reporting symptoms (p<0.001).12) The most frequent symptoms in the primary care cohort were abnormal fatigue (7%), neurological disorders (6.8%), and respiratory disorders (6%). Hospitalized patients more frequently displayed psychological symptoms (36.7%), cardiac involvement (23.3%), and respiratory disorders (18.3%).
Cases vs. Controls
Only six studies included a control group. These studies investigated symptoms in children and adolescents without evidence of SARS-CoV-2 infection.9,14,18,21-22,24. Four showed that persistent symptoms were more prevalent in patients with SARS-CoV-2 infection, but only three were statistically significant.9,21,22,24 Borch and colleagues reported that children in the control group aged 0-5 years experienced significantly more cough, concentration difficulties, and diarrhea than children in the SARS-CoV-2 group (p<0.001).22 Additionally, 6-17-year-old controls were more prone to concentration difficulties, headache, nausea, muscle pain, cough, and diarrhea than SARS-CoV-2 counterparts (p<0.001).22
Discussion
This review showed that the prevalence of Long COVID in children and adolescents was highly variable among studies, ranging from 2 to 66%, which can be explained by the great methodological and inclusion criteria heterogeneity among studies. In addition, some studies used online surveys, which may have caused a bias towards the selection of patients with higher socioeconomic background, who also seem to have a lower risk of poor outcomes after SARS-CoV-2 infection.25
The most common symptoms reported in the studies assessed were fatigue, shortness of breath, headache, sleep disturbance, concentration difficulties, chronic cough, dizziness, myalgia, chest pain, reduced smell or anosmia, abdominal pain, and loss of appetite or weight. The absence of a control group in most studies makes it difficult to ascertain whether these symptoms were only attributable to post-COVID or may have been caused by the pandemic context, due to the negative impact (including in psychosomatic symptoms) that lockdown measures had on children and adolescents. In fact, one of the studies including a control group showed that concentration difficulties, headache, muscle pain, cough, nausea, and diarrhea, which have been reported as Long COVID symptoms, were statistically more significant in the control group.18 This may reflect the highly negative impact and social implications of the pandemic on children’s mental and physical health. However, although that was a population-based study including a seronegative control group ─ which is an important strength ─, it had a short follow-up time and relatively small sample size. On the other hand, studies including a control group have the important limitation of having potentially included children with SARS-CoV-2 infection who did not undergo testing, since children usually have no or only few symptoms of acute COVID-19. This highlights the importance of conducting more controlled studies to understand the real prevalence of persistent symptoms attributed to SARS-CoV-2 infection.
Risk factors for the development of Long COVID identified in this review include older age, muscle pain on admission, ICU admission, history of allergic diseases, higher BMI, and longer duration of infection. The most common risk factor was older age, but most studies did not discriminate between age groups. To clarify this, future studies should stratify patients by age group to assess the impact of this factor on the development of Long COVID. In fact, the higher risk seen in older patients may be related to the fact that younger children might not be able to adequately express their emotional and functional status.
The longest follow-up in the studies assessed was 11 months, although the evidence in adults indicates that symptoms may persist longer than one year. This shows the importance of studies in children with longer follow-up to determine which symptoms are more likely to persist over time.
Lastly, it should be noted that studies may have included different dominant SARS-CoV-2 variants, which might imply a different risk of Long COVID. In the future, it would be interesting to investigate whether different variants can translate into different Long COVID prevalence.
Conclusion
Long COVID represents a significant public health concern, especially in children and adolescents. Investigating its pathophysiology and symptomatology is relevant to establishing appropriate protective measures and rehabilitation programs and implementing specific guidelines. Evidence regarding Long COVID in pediatric patients is still limited, and most studies assessing this subject have several limitations, highlighting the importance of continuously monitoring the impact of the disease in children and adolescents. Appropriate case-control studies are relevant to better understand its true impact in the real-world setting.