Introduction
Neonatal lupus erythematosus (NLE) is considered a model of passively acquired autoimmune disease characterized by the transplacental passage of maternal antibodies, mainly anti-Sjogren’s syndrome A/Ro (anti-SSA/Ro) and anti-Sjogren’s syndrome B/La (anti-SSB/La), and binding of these antibodies to the developing fetal tissues.1,2 Recent publications have also shown the significant role of anti-ribonucleoprotein antibodies (anti-RNP) in NLE.3,4 Antibodies produced by the mother belong to the immunoglobulin G group and enter the fetal bloodstream from the time the placenta is formed, around the 12th gestational week.2,5 These antibodies are directed against autoantigens and may trigger an inflammatory cascade, with relevant consequences for the fetus or newborn, since they are responsible for direct toxic effects on the heart and other organs.2,4,5
Seropositive mothers may have a previously diagnosed disease, like Sjögren’s syndrome, systemic lupus erythematosus (SLE), rheumatoid arthritis, or undifferentiated connective tissue disease, but in 25─60% of cases, they are asymptomatic at the time of childbirth.3,5,6 Half of asymptomatic mothers develop symptoms of immune-mediated disease after a median of three years, reason why some authors suggest their close monitoring after childbirth.4,5 The most common NLE manifestations include skin lesions and congenital heart block (CHB), while hepatobiliary, hematological, neurological, and respiratory signs are much less frequent.3 The incidence of NLE in neonates delivered to mothers with coincident serological profile is approximately 1-2%, with a recurrence rate of about 18─20% in subsequent pregnancies.1,4,5,7 The condition equally affects boys and girls. Children with a history of neonatal lupus are likely at increased risk of autoimmune diseases later in childhood or adulthood, although the magnitude of this risk remains uncertain.4,8,9
Objective
The aim of this review was to summarize the current state of knowledge on NLE, providing clinicians with a reference tool for diagnostic and therapeutic guidance.
Pathogenesis
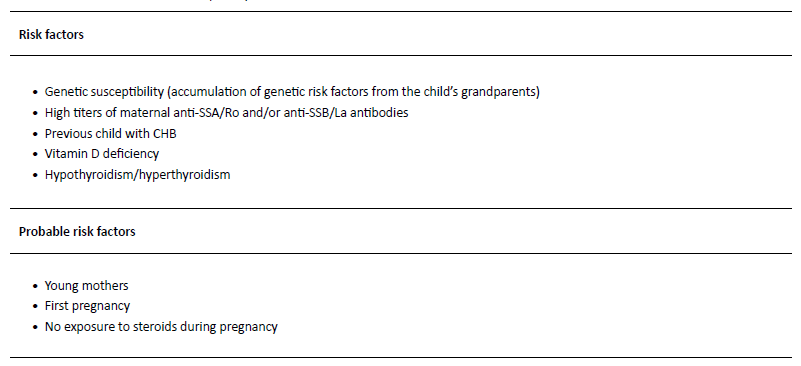
Despite the acknowledgment that NLE onset is related to the transplacental passage of maternal auto-antibodies, 1-3 the pathophysiology of the disease is not yet fully understood.3 In fact, two non-mutually exclusive mechanisms resulting in atrioventricular (AV) block have been proposed. According to one, the physiological process of apoptosis during fetal development results in the expression of intracellular antigens (Ro52 and Ro60) on the surface of fetal cells, particularly cardiac cells.4,10,11 In the second trimester, maternal anti-SSA/Ro and anti-SSB/La antibodies start to cross the placenta, and the antigen-antibody complex is then opsonized and phagocytized, triggering a proinflammatory process with subsequent fibrosis and tissue damage, particularly on the AV node and surrounding tissues.4,10,11 The second hypothesis is based on mimicry, whereby autoantibodies target and cross-react with L-type calcium channels. This could cause an imbalance of calcium homeostasis and fetal heart rhythm disturbances.3,4,10,12-14 While the interaction between maternal autoantibodies and fetal antigens seems crucial, 98% of children exposed to those antibodies are healthy.3 This suggests that additional factors are likely to play a role in the pathogenesis of the disease (Table 1).3,10,11,15,16
Clinical presentation
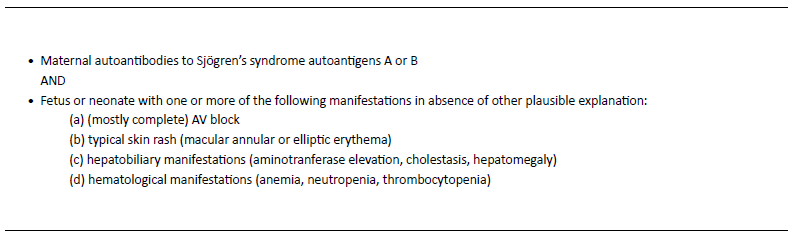
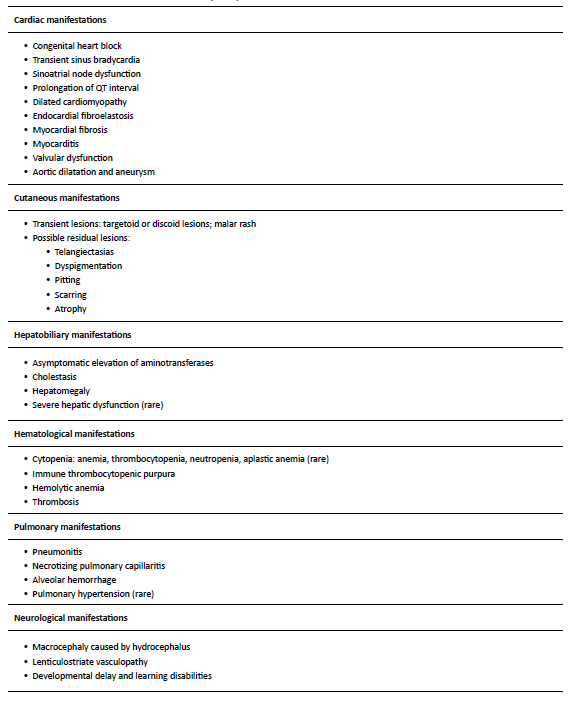
The clinical features of NLE include both reversible and irreversible manifestations.5 Reversible manifestations, like cutaneous lesions, hematological and pulmonary abnormalities, and hepatobiliary dysfunction, disappear spontaneously as the levels of autoantibodies decrease in the bloodstream, generally in the first 6─8 months of age.2,5,17 About 2% of children develop irreversible and potentially life-threatening manifestations, including disturbances of the cardiac stimulatory and conduction systems.2-5 Cardiac manifestations are the only ones that can be detected in the prenatal period. Because of this, NLE diagnosis is established when the mother has anti-SSA/Ro or anti-SSB/La autoantibodies, and the fetus or neonate develops (mostly complete) AV block, or when the neonate develops the typical skin rash or hepatobiliary or hematological manifestations in the absence of other plausible explanations (Table 2).4 Table 3 summarizes the clinical manifestations of NLE.
AV, atrioventricular block
Cardiac involvement
The most commonly reported NLE manifestation is cardiac disease.8 Arrhythmogenic damage to the conduction tissue may lead to congenital AV block, which is the most severe ─ and sometimes lethal ─ NLE manifestation.3,18 Congenital AV block usually develops after 17 weeks of gestation and is frequently detected between weeks 18 and 24, presenting as fetal bradycardia with ventricular rate of 40─60 beats per minute (bpm).3 About 20% of cases are diagnosed in the third pregnancy trimester and only uncommonly in the neonatal period.3,8,19 According to the Brazilian Cardiology Society, in pregnant women with positive anti-Ro/SSA or anti-A/SSB antibodies, the AV interval should be measured weekly from weeks 18 to 26, and myocardial function should be monitored every four weeks until delivery.20
The degree of AV block can be categorized as first-degree, second-degree, or third-degree (complete) block.19 Fetuses with first-degree AV block have a prolongation of AV time (defined as a prolonged PR interval of >200 milliseconds), with 1:1 AV conduction and normal heart rate. Second-degree AV block is defined as failure of conduction of at least one nonpremature atrial impulse to the ventricles. Mobitz type I, or Wenckebach, is the most common type of second-degree AV block and usually involves progressive prolongation of the PR interval until an eventual failure to conduct an atrial beat to the ventricle. A second form of second-degree AV block, or Mobitz type II, is characterized by a constant PP interval without lengthening of the PR interval prior to the nonconducted P. In complete or third-degree AV block, the atrium and ventricle beat independently as a consequence of complete loss of the AV connection (Figures 1 and 2).19 AV block in NLE is usually complete at the time of diagnosis, but first- and second-degree block can also occur and may completely resolve during the first few months after birth.3 However, its severity may also change over time, developing into permanent complete heart block.2,3,5,13 Less frequently, damage to the conduction tissue may lead to transient sinus bradycardia, sinoatrial node dysfunction, prolongation of the QT interval, and Wolf-Parkinson-White syndrome.3 The clinical features of neonates with CHB depend on the effect of heart rate on cardiac output. Low heart rate may result in fetal or neonatal heart failure. Fetal hydrops and intrauterine death may occur in most severe cases. Postnatally, the typical signs of heart failure and low cardiac output manifest in neonates with poor hemodynamic status.2 Some neonates with complete AV block can develop compensatory mechanisms, although most require pacemaker implantation.2 Although NLE cardiac manifestations typically occur in utero or in the neonatal period, Rumancik et al. reported a case of “late-onset” neonatal lupus on a four-week-old girl presenting with severe dilated cardiomyopathy, dyskinetic ventricular septum, and left bundle branch block, highlighting the need for long-term cardiac follow-up of patients born with neonatal lupus, even if cardiac manifestations are lacking in the peripartum period.7 This case highlights the need for long- term cardiac follow-up of patients born with neonatal lupus, even if cardiac manifestations are lacking in the peripartum period.
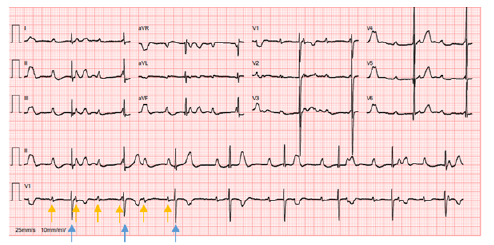
Figure 1 Neonatal complete atrioventricular block, with auricular rate of 100 bpm and ventricular rate of 49 bpm with p waves (yellow arrows) dissociated from QRS complexes (blue arrows)
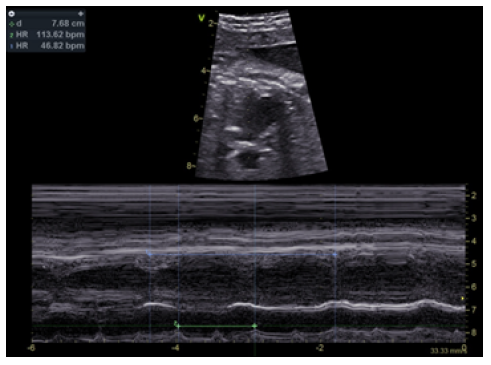
Figure 2 Fetal echocardiogram showing complete atrioventricular block, with auricular rate of 113 bpm and ventricular rate of 46 bpm
Although structural abnormalities are uncommon findings, NLE may also have an impact on the myocardium and endocardium. The spectrum of structural cardiac manifestations of neonatal lupus includes endocardial fibroelastosis, myocarditis (which can progress to dilated cardiomyopathy), and valvular abnormalities.11,18 Fibroelastosis appears to result from an inflammation cascade triggered by maternal antibodies. Its key finding is an echogenic endocardium along the left ventricle, characterized by diffuse thickening secondary to proliferation of fibrous and elastic tissue.13,19 Myocarditis has also been reported in some patients with SLE and congenital atrioventricular block.4,11,21,22 Dilated cardiomyopathy may include bilateral ventricular dilatation and reduced ejection fraction, usually occurring in association with heart block and often being life-threatening.8,23 Valvular anomalies may include fibrosis involving the mitral valve papillary muscles and fusion of the chordae tendineae of the tricuspid with AV valve dysfunction.19,23 Akbariasbagh P. et al. reported the rare case of a preterm male who presented with dilatation of the ascending aorta and consequent formation of aortic aneurysm, which was thought to be caused by inflammation in the aortic adventitia associated with the transplacental passage of maternal antibodies.9
Cutaneous lesions
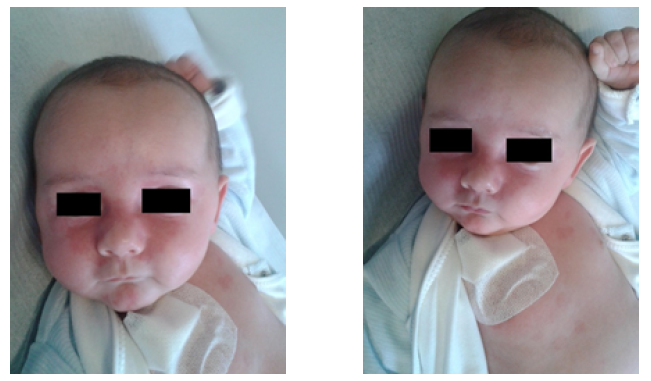
Cutaneous manifestations are present in approximately 40% of NLE cases.3 Although eruptions are characteristic, they are sometimes misdiagnosed as birth trauma, fungal infection, or eczema, especially in newborns from asymptomatic mothers.24 Lesions are usually not apparent at birth and develop after the first three months of life, mostly following sunlight exposure.3,4 The cutaneous hallmark of NLE is a superficial inflammatory rash affecting the upper eyelids, especially the periocular area, inducing the characteristic “eye mask” or “raccoon-like” appearance. The neck, perioral, zygomatic and temporal areas, and less frequently, the trunk or extremities may be involved (Figure 3).3,25 It usually presents as macular annular or elliptic erythema, but plaque-like lesions may also be observed. Borders are regular and sometimes raised, and central clearing is observed in about 30% of cases. Lesions usually have 1 cm in diameter but may coalesce and form larger erythematous areas.4,25 Severely affected areas may sometimes blister or crust. Persistence of telangiectasias, hyperpigmented skin areas, and skin atrophy have been reported in about 20% of cases.4 Although usually not required, histologic examination commonly reveals interface dermatitis, with granular deposition of immunoglobulin G at the dermo-epidermal junction.4 Despite this, some case reports have described typical annular erythematous macules or plaques with interstitial infiltration of mononuclear cells mixed with segmented neutrophils.26,27 These cases indicate that NLE should be included in the differential diagnosis of histiocytoid neutrophilic dermatitis and neutrophilic dermatitis,26,27 as well as other cutaneous lesions, like atopic dermatitis, seborrheic dermatitis, tinea capitis, eyelid telangiectasias, erythema multiforme, and familial annular erythema.3,28
Hepatobiliary manifestations
Liver damage can be isolated or secondary to heart failure in the context of congenital heart block.13 Although rarely reported and potentially underestimated, its prevalence ranges between 9% and 27%, usually accompanying cutaneous abnormalities.3,13 The most common manifestations, which are mild and transient, include cholestasis, possibly associated with hepatic cytolysis manifesting in the first weeks of life, and moderate isolated hepatic cytolysis occurring around two to three months of life, possibly associated with hepatomegaly.13,29 In cases of severe hepatic dysfunction, biopsy reveals mild bile duct obstruction, portal fibrosis, and sporadic giant cell transformation resembling idiopathic neonatal giant cell hepatitis.22,29
Hematological findings
Anemia, neutropenia, thrombocytopenia, and rarely aplastic anemia occur in approximately 20% of NLE cases.4,30 Cytopenia pathogenesis is most probably related to the suppressive effect of maternal antibodies on the bone marrow rather than to increased peripheral destruction of blood cells.4,13 However, one case of hemolytic anemia with positive direct antiglobulin test has been described.31 Other rare manifestations include immune thrombocytopenic purpura, microangiopathic hemolytic anemia, and thrombosis stemming from the transplacental passage of antiphospholipid antibodies.3
Pulmonary involvement
Pulmonary NLE manifestations have rarely been reported in the literature. Most neonates with circulating maternal antibodies have no lung manifestations or only present mild tachypnea or hypoxemia. However, respiratory manifestations may be significant in some cases.32 Pneumonitis, pleural effusion, necrotizing pulmonary capillaritis, and alveolar hemorrhage have been described as clinical manifestations of pulmonary involvement in neonatal lupus (Figure 4).3,34-36 Pereira S. et al. described a clinical case of severe pneumonitis diagnosed on day 17 of life in a neonate with complete heart block and definitive pacemaker.33 The neonate presented with acute severe respiratory failure requiring mechanical ventilation and oxygen therapy, with diffuse infiltrates in chest radiograph and ground glass-like computed tomography (CT), which responded to intravenous methylprednisolone pulses followed by oral prednisolone. Pulmonary hypertension associated with respiratory disease has also been reported as a rare neonatal lupus manifestation. Maltret A. et al. described the clinical presentation, management, and outcome of a series of four neonates who developed reversible pulmonary hypertension associated with autoimmune congenital complete heart block.34 The diagnosis was suspected on transthoracic echocardiography at a median of 42 days of life (range 10-58) and confirmed by right heart catheterization in all neonates. All had some degree of hypoxemia and respiratory distress reversible under oxygen and nitric oxide. Lung CT disclosed ground glass anomalies in all. Management included steroid therapy in three patients, associated with sildenafil in two. Pulmonary hypertension resolved at a median of four weeks after treatment start in the three neonates who received treatment, and after one year in the neonate who did not receive specific treatment.
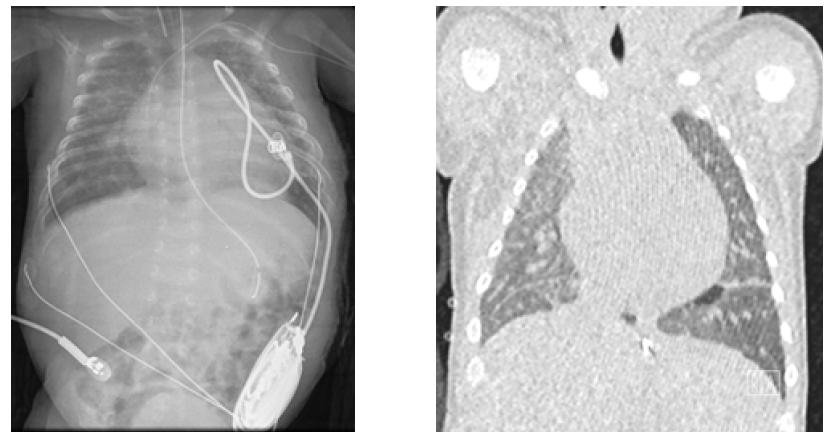
Figure 4 Pulmonary involvement with multiple focus of neonatal lupus pneumonitis depicted on chest radiography (left) and computed tomography (right)
Neurological manifestations
Most newborns show no clinical neurological symptoms at birth.3 Rare neurological abnormalities may appear during the first year of life and present as macrocephaly with or without associated hydrocephalus. Other potential findings described include lenticulostriate vasculopathy, ventriculomegaly, and possible dysgenesis of structures supplied by the lenticulostriate vasculature.4,13 Some clinical manifestations may also be observed later in infants’ life. Children may develop seizures, developmental delay and learning disabilities, particularly dyslexia, attention deficit, hyperactivity, and obsession and compulsion disorders.3
Management
Prenatal screening and in-utero management
Pregnant women who test positive for anti-SSA/Ro or anti-SSB/La autoantibodies are considered to have a risk pregnancy.4,22 The increased risk period for the development of fetal heart injury is between 18 and 26 weeks of pregnancy and it can be detected by fetal echocardiography.18 This is a safe and non-invasive method for assessment of cardiac structure and rhythm and should be provided prenatally if the mother tests positive for specific antibodies.3,19,35 If maternal autoantibodies are positive and the fetus is in sinus heart rhythm, weekly measurements of the AV interval (mechanical PR interval) are recommended from 18 weeks onwards. If mechanical PR remains stable below 150 ms after week 26 - and given the rarity of de novo heart block after this stage - the current recommendations are for myocardial function monitoring every four weeks from week 26 until delivery.20,36 Conversely, if a progressive mechanical PR increase over 150 ms is noted from week 18, weekly follow-up until week 26 and every two weeks afterwards is recommended.18,20 Although controversial, treatment with dexamethasone at a dose of 4-8 mg orally can be started in cases in which AV interval is >150 ms or progressively increasing.20
Irreversible third-degree CHB is the most serious NLE manifestation. Although rare, it is associated with increased risk of intrauterine fetal death, neonatal morbidity and mortality, and long-term sequelae,18,35 with most surviving children requiring permanent pacemaker implantation.8,19 Fetal bradycardia is the main finding and often the only one.18 Immune-mediated AV block in NLE may benefit from in-utero treatment with fluorinated glucocorticoids, which are not inactivated by placental 11-β dehydrogenase.19,35,37 The rationale for this type of management is achieving improved fetal outcomes by reducing the circulation and transfer of maternal antibodies and cardiac tissue inflammation before fibrosis and irreversible CHB occur.18 Reported benefits of dexamethasone (4-8 mg/d) include decreased inflammation, incomplete block reversal or stabilization, and hydrops or endocardial fibroelastosis improvement or resolution.20,21,37,38 No therapies have been shown to reverse third-degree block (including glucocorticoids, apheresis, intravenous immunoglobulin [IVIG], or hydroxychloroquine).38-40
Despite the potential risks of dexamethasone, like growth restriction, oligohydramnios, or gestational diabetes, its use may be considered in cases of second- or first-degree AV block, particularly in the presence of additional cardiac findings of inflammation (echogenicity, valve regurgitation, cardiac dysfunction, and/or effusion), as a way of preventing progression to complete heart block.17,37 IVIG is also an option.41 Fetuses usually tolerate ventricular rate >55 bpm in the absence of concomitant anomalies. The use of maternal beta-agonist therapy (e.g., terbutaline) when fetal heart rate is < 50─55 bpm has been shown to increase the heart rate and stroke volume in small case series.42,43 Although this approach has not been validated in comparative studies, several centers use it when fetal heart rate is <50 bpm. Fluorinated glucocorticoids and/or IVIG can be used in presence of extranodal disease (e.g., cardiomyopathy, endocardial fibroelastosis, myocarditis), even in absence of complete heart block. However, the effectiveness of these agents in endocardial fibroelastosis treatment is still unclear.44-46
Pregnant women with systemic rheumatic diseases should be followed in tertiary centers and receive adequate treatment with hydroxychloroquine aiming at disease remission. Several retrospective studies have suggested that hydroxychloroquine decreases the overall risk of cardiac lupus, as well as the risk of cardiac involvement in fetuses with prior history of cardiac lupus in a sibling.20,47,48 In fact, most authors recommend hydroxychloroquine to all positive anti-SSA/Ro pregnant women, due to its effect in reducing the risk of NLE recurrence in subsequent pregnancies (up to 20% of cases).18 Therefore, regardless of the maternal health status, hydroxychloroquine should be initiated at the dose of 5 mg/kg daily between the 6th and 10th weeks of gestation in seropositive women not already taking the medication (including in pre-conceptional setting) as a way to optimize the exposure by 16 weeks of gestation. In the pre-conceptional period, hydroxychloroquine may be prescribed 2-8 weeks before the planned pregnancy, as the evidence shows that it can reduce the incidence of NLE, even in asymptomatic cases.49 Asymptomatic mothers diagnosed following NLE diagnosis in the offspring should be referred to Rheumatology follow-up, since the risk of developing symptomatic disease is high. Preemptive treatment for future pregnancies is also paramount.4 A suggested approach to these cases is summarized in Figure 5.18,20,21,37
Overall, the treatment options for complete heart block in-utero are limited, and management is primarily expectant, based on monitoring of feto-maternal health status and screening of extranodal disease. Fortunately, most fetuses tolerate slow heart rhythm.4 The indications for delivery should be determined according to gestational age and severity of fetal manifestations. In fetuses with significant hydrops, progression of fetal heart failure (specifically an increase in the amount of pleural fluid, ascites, or pericardial effusion), and ventricular rate <50 bpm, early delivery and emergency pacing may be needed. Other fetal indications include severe oligohydramnios and intrauterine growth restriction.20,21
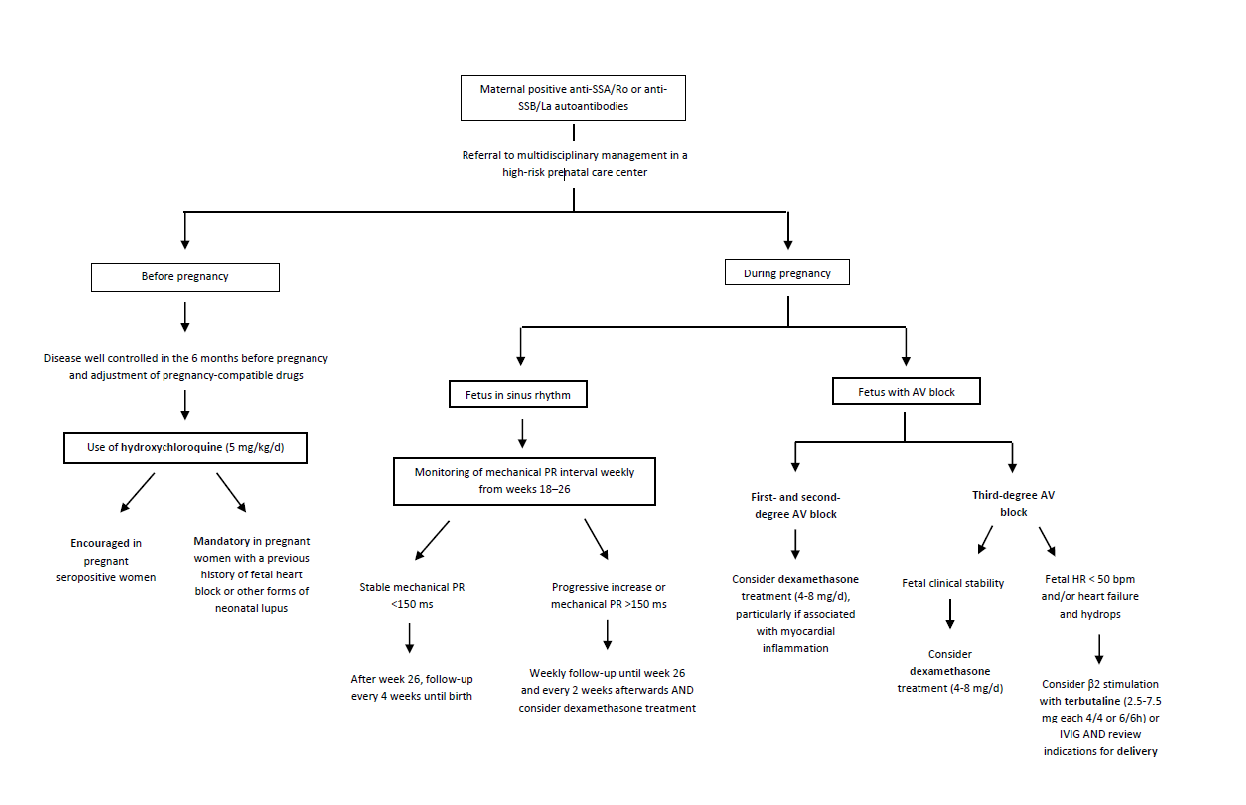
Figure 5 Flowchart of the suggested approach for pregnant women with positive. anti-SSA/Ro or anti-SSB/La antibodies. anti-SSA/Ro, anti-Sjogren’s syndrome A/Ro antibody; anti-SSB/La, anti-Sjogren’s syndrome B/La antibody; AV, atrioventricular block; bpm, beat per minute; d, day; h, hour; HR, heart rate; IVIG, intravenous immunoglobulin; kg, kilogram; mg, milligram, ms, millisecond
Postnatal approach
Infants affected with NLE should be managed in a tertiary care center, where different clinical subspecialties are available.4 It should be clarified that postnatal development of NLE is independent of breastfeeding, and thereby this practice should not be discouraged even if the maternal serological profile is compatible with NLE diagnosis.3 Specialized management depends on the type and severity of manifestations.3,4,13,19,49 Cardiac involvement is associated with poor prognosis, especially if any of the following is present: gestational age <20 weeks at diagnosis, ventricular rate <55 bpm, hydrops fetalis, impaired left ventricular function, cardiomegaly, AV valve regurgitation, endocardial fibroelastosis, or low aortic flow velocity.11,50,51 In most cases, the main decision is determining the need and timing of permanent pacemaker insertion, as more than 90% of patients will ultimately require it.4,11 Regarding the maternal approach, testing for maternal anti-Ro/SSA antibodies should be performed in mothers of all neonates with heart block and no identified causal structural abnormality since these antibodies account for 80─95% of the reported CHB cases in the fetus and neonate. Infants up to eight months of age with annular or polycyclic rash and/or heart block of any degree should also be tested for maternally derived antibodies (although de novo development of CHB after birth is extraordinarily rare and may indeed represent evolved first- or second-degree block missed in utero). A positive test in the child or mother fulfills the diagnostic criteria for neonatal lupus.36
Prognosis
The prognosis of NLE depends on clinical manifestations. In cases of isolated non-cardiac abnormalities, patients rarely require treatment, and the prognosis is very good. The presence of cardiac involvement is associated with less favorable prognosis. The mortality rate in complete heart block is about 20% (10─29%), and 63─93% of surviving patients require pacemaker implantation.3 The outcome for patients diagnosed as neonates is better than for those diagnosed in utero. Infants and young children with CHB who are asymptomatic usually remain so until later childhood, adolescence, or adulthood.52
Conclusion
NLE is an uncommon disease associated with maternal autoantibodies against proteins of the Ro/La (SSA/SSB) family. The most common clinical findings include complete heart block and cutaneous lesions, but some children present with cardiomyopathy, hepatobiliary disease, or hematological, pulmonary, or neurological findings. As only a minority of babies exposed to autoantibodies develop the disease, additional factors are likely to be important in determining disease expression.
Early and multidisciplinary management of infants affected with NLE in a tertiary care center is recommended. Postnatal pacemaker insertion may be indicated, and erythrocyte or platelet transfusions, corticosteroids, or IVIG may be indicated to treat severe manifestations. The prognosis depends on clinical manifestations.