Introduction
Urinary tract infections (UTIs) are common in children and are a frequent reason for Emergency Department (ED) visits.1,2 The decision to start empirical or prophylactic antibiotic therapy should be based on factors such as age, clinical condition, and the most commonly isolated bacteria in urine cultures (UCs), and should be adapted to the antimicrobial susceptibility profile of the pediatric population in each geographic region.1,3,4
UTIs are more common in females of all ages, except during the first three months of life, when they are more common in males.2
UTIs are defined by the presence of a significant number of microorganisms in aseptically collected urine associated with concordant clinical symptoms.1,3,4 Most UTIs are caused by bacteria, with Escherichia coli accounting for approximately 70-90% of all cases..2,4) Other common uropathogens include Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterococcus spp, and Staphylococcus saprophyticus, the latter being more common in UTIs in female adolescents.2
The diagnosis requires urine collection using an aseptic technique, with growth of bacteria in sufficient numbers in the UC.1-3) In Portugal, according to Direção-Geral da Saúde (DGS) Regulation 008/2012, urine samples are considered positive with growth of ≥ 1 colony-forming unit (CFU)/mL by suprapubic aspiration (SPA); ≥ 104-105 CFU/mL by catheterization; or ≥ 105 CFU/mL by midstream urine (MSU).3
When UTI is clinically and analytically suspected, it is imperative to initiate antibiotic therapy immediately after urine collection for UC.1-3 The choice of empirical antibiotic therapy depends on factors such as patient age, clinical presentation, and local antimicrobial susceptibility patterns.3 In addition, the presence of known renal or urinary system abnormalities, previous antibiotic therapy, and ongoing prophylactic antibiotic therapy (treatment with a different antibiotic) should be considered.3
The empirical antibiotic therapy of choice for UTIs in children includes cefuroxime and amoxicillin-clavulanic acid.3 In female adolescents with cystitis, nitrofurantoin or fosfomycin may be an option.3,5) These recommendations exclude neonates and patients with previously known renal or urinary system abnormalities.3
The main complication of lower UTI (cystitis) is its spread to the upper urinary tract, causing pyelonephritis. Some pyelonephritis, especially those occurring in the first years of life, may be complicated by lesions of the renal parenchyma, which may later evolve to renal scarring and progressive kidney dysfunction. In some situations, namely in children with recurrent UTIs, prophylactic antibiotic therapy may be indicated, with trimethoprim-sulfamethoxazole and nitrofurantoin being the recommended antimicrobials for this purpose.3,6
The indiscriminate and excessive use of antimicrobial agents, facilitated by their easy accessibility, has led to the emergence of resistant and multi-resistant bacteria, which has become a major public health problem today.1,4-12 Therefore, it is essential to promote the rational use of antimicrobial agents at the community level.8,11,12
One of the consequences of the overuse of broad-spectrum antibiotics is the increase in the prevalence of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, which are responsible for a growing number of cases of UTIs in children.4,9,11-12) Escherichia coli and Klebsiella pneumoniae are the most common causative organisms.4,9,13-15 The majority of ESBL-producing Enterobacteriaceae are resistant to the most commonly used antimicrobial agents.9,10,14) To overcome this resistance, combinations of beta-lactams and beta-lactamase inhibitors, as well as broader-spectrum antibiotics, have been used. According to the latest Infectious Diseases Society of America guidelines, ESBL-producing Enterobacteriaceae are universally susceptible to carbapenems, making these agents the treatment of choice for pyelonephritis and complicated UTIs. Alternatively, trimethoprim-sulfamethoxazole or fluoroquinolone can be used. The first-line treatment for cystitis includes nitrofurantoin or trimethoprim-sulfamethoxazole, while the second-line treatment includes amoxicillin-clavulanic acid, fosfomycin, or an aminoglycoside. Piperacillin-tazobactam therapy is not recommended.15
In most cases of UTI, initial antimicrobial therapy is empirical, and this choice should be based on the prevalence of pathogens and their susceptibility to antimicrobial agents. Given the large geographic variability of both parameters, epidemiologic surveillance studies conducted by each health unit are crucial for prescribing empirical therapy.8,16
The latest Clinical Microbiology consensus, published in 2017, recommended that antimicrobial susceptibilities be presented in microbiology charts using the color green for susceptibility greater than 80%, yellow for susceptibility between 50 and 80%, and red for susceptibility less than 50%.8 Therefore, a susceptibility rate of 80% seems to be the acceptable susceptibility threshold for initiating empirical antibiotic therapy.
The latest updates of the expected susceptibility phenotypes for the different bacterial species were published by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) in 2022.17) According to EUCAST, susceptibility patterns for fosfomycin and nitrofurantoin should only be reported for uncomplicated UTIs caused by Escherichia coli and are not recommended for other Enterobacteriaceae.17,18
The aim of this study was to analyze the UC results of urine samples collected using an aseptic technique in the pediatric ED of a level II hospital in Portugal between January 2019 and December 2021 with the purpose of characterizing the pediatric population diagnosed with UTIs and analyzing positive UC results to identify the most frequently isolated bacterial agents and analyze their antimicrobial susceptibility profile. The ultimate goal was to optimize the use of empirical and prophylactic therapy.
Material and methods
This was a retrospective, descriptive study of all UC results of children and adolescents referred from the Pediatric ED to the Clinical Pathology Department of a level II hospital between January 2019 and December 2021. All urine specimens collected by aseptic non-touch (drainage bag) or unknown technique or by puncture catheterization in patients with chronic catheterization were excluded. All UCs with isolation of a single agent in colony count ≥ 1 CFU/mL by SPA, ≥ 104 CFU/mL by catheterization, and ≥ 105 CFU/mL by MSU were considered positive.3
Regarding laboratory procedures, urine samples were inoculated onto cystine-lactose-electrolyte-deficient (CLED) agar culture and incubated in an aerobic atmosphere at 35°C for 18-24 hours, after which bacterial growth was assessed. From January 2019 to December 2019, isolated colonies were identified using the Vitek®2 automated identification system. From January 2020, identification of these isolates was performed using MALDI-TOF (MALDI Biotyper®).
The Vitek®2 system was used to determine the minimum inhibitory concentration of the isolates and the susceptibility was interpreted according to the current EUCAST standards.17,18
Susceptibilities to the following antimicrobials were provided and analyzed: ampicillin, oxacillin, amoxicillin-clavulanic acid, cefuroxime, ceftazidime, fosfomycin, nitrofurantoin, trimethoprim-sulfamethoxazole, piperacillin-tazobactam, and vancomycin.
Data analyzed included patient age and sex, collection technique, isolated microorganism, isolation of ESBL-producing Enterobacteriaceae, and antimicrobial susceptibility pattern.
Statistical analysis was performed using IBM SPSS version 26.0. Categorical variables were presented as absolute (n) and relative (%) frequencies. Collection techniques were defined as SPA, catheterization, or MSU. The antimicrobial susceptibility pattern was classified as standard dose susceptibility (S), increased exposure susceptibility (I), and resistance (R) according to the EUCAST classification in effect since 2019.19 The only continuous variable in study was age, defined in years and characterized by mean and median. All children and adolescents aged between one month and 17 years were included.
The study was approved by the Ethics Committee of the participating hospital.
Results
During the study period, the Clinical Pathology Department received and analyzed 3222 urine specimens from the pediatric ED, of which only 774 (24%) were collected using an aseptic technique and were positive, according to previously defined criteria. The urine collection techniques are shown in Table 1.
The 774 UCs included in the study were from a total of 610 patients, 73.8% of whom were female, with a mean age of five years and a median age of three years; 49% were children up to two years of age.
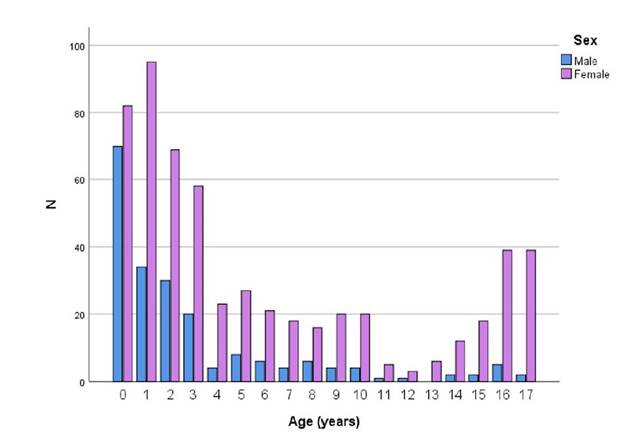
The distribution of positive UCs by age and sex is shown in Figure 1. There was a predominance of UTIs in females of all ages, and a higher prevalence of UTIs during the first year of life, followed by a significant decrease (especially after four years of age) in males.
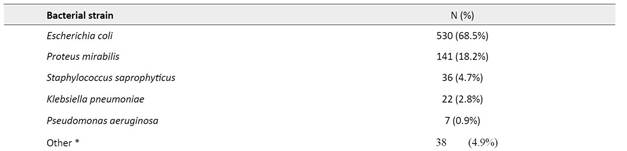
Eighteen different bacterial strains were identified in positive UCs (Table 2). The most commonly isolated etiological agents were Escherichia coli (68.5%), Proteus mirabilis (18.1%), and Staphylococcus saprophyticus (4.7%), followed by Klebsiella pneumoniae (2.8%) and Pseudomonas aeruginosa (0.9%).
The distribution of bacterial strains by age group is shown in Table 3. Escherichia coli was the most frequently isolated etiological agent in all age groups. In the group of female adolescents aged between 14 and 17 years, most UTIs were caused by Escherichia coli (52.9%) and Staphylococcus saprophyticus (26.8%).
The antimicrobial susceptibility pattern of the five most common etiological agents is shown in Table 4.
Ten cases (1.3%) of ESBL-producing Enterobacteriaceae were identified, and this phenotype was detected in 1.5% of Escherichia coli (n=8) and 9% of Klebsiella pneumoniae (n=2) cases. ESBL-producing Escherichia coli were identified in all age groups, with 50% of cases in children less than two years of age. Klebsiella pneumoniae was identified only in adolescents between 14 and 17 years of age. The susceptibility pattern of these bacteria to recommended antibiotics is shown in Table 5.16 The susceptibility pattern to fluoroquinolones and carbapenems was not available and therefore not analyzed in this study.
Discussion
In this study, Escherichia coli was the most frequently isolated etiological agent in all age groups, which is consistent with data from other studies.2,4 This agent showed a resistance profile of 26.2% to amoxicillin-clavulanic acid, which exceeds the resistance threshold considered acceptable. According to the latest Clinical Microbiology consensus, when the resistance profile is greater than 20%, the empirical use of the antibiotic in infections caused by the identified etiological agent should be questioned.8 Thus, according to this study’s results, amoxicillin-clavulanic acid should not be recommended as first-line empirical therapy for the pediatric population in the region. Cefuroxime resistance rate was 4.9% and should therefore be recommended as the empirical antibiotic of choice.
Proteus mirabilis has a suitable susceptibility profile for all empirical antibiotics used for UTIs in different age groups.
Staphylococcus saprophyticus was the third most frequently isolated microorganism in this study. Oxacillin screening allows to know the susceptibility to isoxazolyl penicillins, penicillin beta-lactamase inhibitors, and cephalosporins. The analysis showed over 80% susceptibility to oxacillin, which means that amoxicillin-clavulanic acid and cefuroxime can still be used as empirical antibiotic therapy for UTIs caused by Staphylococcus saprophyticus. Likewise, it is 100% susceptible to nitrofurantoin, which makes this antibiotic a suitable option for UTIs without fever in female adolescents, as indicated in DGS Rule 008/2012.3 On the other hand, Staphylococcus saprophyticus has an expected resistance phenotype to fosfomycin, so its prescription as first-line empirical antibiotic therapy for UTIs in female adolescents should be discouraged.
Klebsiella pneumoniae is intrinsically resistant to ampicillin. According to this study, it has less than 80% susceptibility to amoxicillin-clavulanic acid (77.3%) and trimethoprim-sulfamethoxazole (72.7%), which is why these antibiotics are not recommended as empirical therapy for infections caused by this microorganism.8 However, it has an adequate susceptibility profile to cefuroxime (90.9%), so this agent should still be used as the empirical antibiotic of choice.
Pseudomonas aeruginosa is rarely responsible for UTIs in children. This microorganism shows intrinsic resistance to amoxicillin-clavulanic acid and cefuroxime and 100% susceptibility to piperacillin-tazobactam and ceftazidime. In the present study, seven cases (0.9%) of UTIs caused by this bacterium were identified in a total of five patients. Therefore, the empirically initiated antibiotic should be changed if this organism is isolated in culture, even before the result of antimicrobial susceptibility testing is known, since it is intrinsically resistant to amoxicillin-clavulanic acid and cefuroxime.
Although a significant number of cases of Escherichia coli with ESBL-producing phenotype were isolated, this phenotype was proportionally more common in Klebsiella pneumoniae strains, which is in line with data retrieved in other studies.10,13 In this study, these bacteria showed a high resistance profile to amoxicillin-clavulanic acid and cefuroxime. Escherichia coli with an ESBL-producing phenotype showed a 100% susceptibility profile to fosfomycin and nitrofurantoin. Therefore, if ESBL-producing Enterobacteriaceae are identified in culture before the result of antimicrobial susceptibility testing is available, the recommendation is to switch therapy to the recommended antimicrobials, namely fosfomycin, nitrofurantoin, or carbapenems.15 However, alarmingly, the susceptibility profile for trimethoprim-sulfamethoxazole was much lower than 80% for both bacterial strains, so its use as first-line empirical antibiotic therapy for the treatment of UTIs in the pediatric population of the region should be discouraged in these cases .8,15
Regarding antibiotic prophylaxis, Escherichia coli was found to have a susceptibility profile greater than 80% for both trimethoprim-sulfamethoxazole (84.7%) and nitrofurantoin (99.8%). Proteus mirabilis and Staphylococcus saprophyticus were susceptible to trimethoprim-sulfamethoxazole in 89.4% and 100% of cases, respectively. In contrast, Klebsiella pneumoniae was susceptible to this antibiotic in only 72.7% of cases.
Conclusions
The present study showed that cefuroxime maintains an adequate susceptibility profile for all microorganisms and should therefore continue to be recommended as the empirical antibiotic of choice for UTIs in the pediatric population of the geographical area considered. Escherichia coli showed a high rate of resistance to amoxicillin-clavulanic acid, exceeding the acceptable resistance threshold for empirical antibiotic therapy. Staphylococcus saprophyticus was the second most common cause of UTIs in female adolescent. However, it showed an expected resistance phenotype to fosfomycin, so its prescription as empirical antibiotic therapy in this group should be discouraged. Trimethoprim-sulfamethoxazole and nitrofurantoin remain appropriate prophylactic antibiotics in most cases.
Establishing appropriate empirical and prophylactic antibiotic therapy for UTIs requires periodic assessment of the susceptibility of the most common etiological agents to various antimicrobials. Since there is variability in the pattern of antibiotic susceptibility between different age groups and geographic regions, periodic updating of this information is essential not only for choosing the most appropriate empirical treatment, but also for controlling and preventing the emergence of bacterial resistance to antibiotics.
List of abbreviations
Acknowledgements
The authors acknowledge the staff of the Department of Pediatrics who contributed to this work.
Authorship
Paula Santos - Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing - original draft
Ana Sofia Nascimento- Data curation; Formal analysis; Investigation; Methodology; Writing - original draft
Filomena Santos - Data curation; Formal analysis; Investigation
Catarina Ribeiro - Conceptualization; Supervision; Validation; Writing - review & editing
Julieta Morais - Validation; Writing - review & editing