Introduction
Caffeine has been consumed in stimulating beverages for centuries, but the active agent in coffee or tea was not discovered until the 19th century.1 In 1819, 25-year-old chemist Friedlieb Ferdinand Runge extracted the first pure caffeine molecule from Arabian mocha beans.2
In the 20th century, caffeine and two structurally related molecules, theophylline and aminophylline, became known as “methylxanthines” and were incorporated into everyday medical practice. These drugs were prescribed for conditions such as asthma, chronic obstructive pulmonary disease, and apnea of prematurity.3
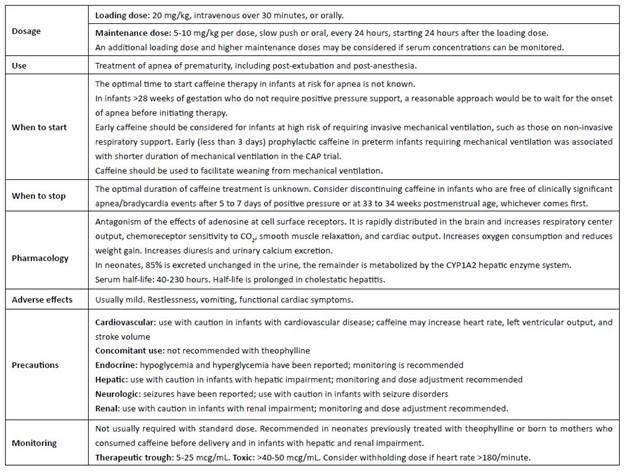
The first use of methylxanthines to treat irregular breathing was described by Vogl in 1927 in adults with Cheyne-Stokes respiration.4 In 1973, Kuzemko and Paala first introduced a methylxanthine into neonatology, using aminophylline to treat apnea of prematurity in a small cohort of preterm infants.5 In the mid-1970s, Aranda and colleagues first used caffeine to treat apnea of prematurity in low-birth-weight infants.6 By measuring plasma levels, they documented the markedly prolonged half-life of caffeine in preterm infants compared to the adult population and proposed a regimen that included a loading dose of 20 mg/kg of caffeine citrate followed 24 hours later by a maintenance dose of 5-10 mg/kg/day of caffeine citrate.6 According to the results of the Caffeine for Apnea of Prematurity (CAP) trial, this regimen is still the most commonly used in neonatal units around the world.7 In the following decades, caffeine became one of the most commonly prescribed drugs in neonatal medicine.8 The World Health Organization has included caffeine citrate in its core list of essential drugs “administered to the neonate”.9
Objectives
The aim of this narrative review was to gather the most relevant information available in MEDLINE on the use of caffeine in preterm infants to help clinicians make the most appropriate use of this therapeutic agent.
Pharmacokinetics and pharmacodynamics of caffeine in neonates
Traditional pharmacokinetic studies typically require frequent, repeated blood sampling and are not easily performed in very small-for-gestational-age preterm infants due to blood volume limitations. Alternative methods using sparse sampling and nonlinear mixed effects modeling (NONMEM) population pharmacokinetics (popPK) have been implemented in caffeine pharmacokinetic studies in neonates.10
Caffeine, or 1,3,7-trimethylxanthine (C8H10N4O2), exhibits rapid gastrointestinal absorption in preterm infants, reaching therapeutic concentrations within 30-60 minutes after oral administration.11-13 Thus, caffeine can be administered parenterally or orally with equal effectiveness without dose adjustments. Neither breast milk nor formula interfere with the gastrointestinal absorption of caffeine.13
Caffeine is rapidly distributed to the brain, and central nervous system levels approximate peripheral plasma levels. Caffeine increases respiratory center output, chemoreceptor sensitivity to CO2, smooth muscle relaxation, and cardiac output. Oxygen consumption may increase and weight loss may occur. Renal effects of caffeine include diuresis and increased urinary calcium excretion.10
Caffeine exerts its pharmacological effects by antagonizing adenosine receptors, and its side effect is the inhibition of phosphodiesterases, resulting in the accumulation of cyclic adenosine monophosphate (cAMP) and potentiating the effects of epinephrine, norepinephrine, and dopamine.10,14-19 Adenosine receptors (A1, A2A, A2B, and A3) activate intracellular G-proteins and exert effects on several signaling molecules, including cAMP, IP3 (inositol triphosphate), arachidonate, choline, and IP3/DAG (diacylglycerol).20,21) Adenosine inhibits adenyl cyclase via high-affinity (AI) receptors and stimulates adenyl cyclase via low-affinity (A2A) receptors.22 These adenosine receptors are found in the brain, respiratory, cardiovascular, and gastrointestinal systems, as well as in the kidneys and endocrine and adipose tissues.10 In general, adenosine acts presynaptically to inhibit neurotransmitter release of acetylcholine, norepinephrine, dopamine, serotonin, and GABA.10 In addition, adenosine decreases spontaneous firing of neurons in the brain and produces sedative and anticonvulsant effects. Caffeine competitively inhibits A1 and A2A receptors, thereby increasing neuronal firing, releasing epinephrine, norepinephrine, dopamine, and serotonin in the brain, and increasing circulating catecholamines.19,23,24
Initial adenosine antagonism with caffeine has a threshold of less than 10 μmol (1.94 mg/L) and may be as low as 2 μmol (0.38 mg/L).16,19 Plasma concentrations in this range (2-3 mg/L) after a 2.5 mg/kg i.v. dose of caffeine citrate are associated with improved respiratory control in preterm infants with apnea, as evidenced by fewer breathing cessations and a more regular breathing pattern.25,26) Therefore, the threshold plasma concentration that represents the lower limit of the therapeutic range for respirogenesis is at least 2 mg/L.10 The ventilatory response and inspiratory drive (VT/Ti) continue to increase as plasma caffeine concentrations reach 5 mg/L and plateau at plasma caffeine concentrations below 20 mg/L.25,26 While doses achieving these plasma concentrations have been repeatedly associated with reduced apnea frequency, higher doses are required to achieve apnea control in some infants.10 Charles et al. reported plasma caffeine concentrations ranging from 18.9 to 79.8 mg/L (mean: 47.4 mg/L) and 4.8-25.1 mg/L (mean: 14.7 mg/L) in infants randomized to daily maintenance doses of caffeine citrate of 20 mg/kg/day or 5 mg/kg/day, respectively.27 The higher dose, and consequently higher plasma drug concentrations, significantly decreased failure to extubate and duration of mechanical ventilation.28
The standard and commonly prescribed doses of caffeine citrate (20 mg/kg loading and 5-10 mg/kg/day maintenance) exert their pharmacodynamic effects on respiratory control and reduce apnea of prematurity (Table 1).10) At higher doses, caffeine can interact with many molecular targets and produce adverse pharmacologic effects. In preterm infants receiving high doses of caffeine (30 mg/kg/day), plasma caffeine levels exceeded these concentrations by approximately 30-fold. At these supra-pharmacologic doses, seizures and other neurologic and cardiovascular adverse events may occur due to GABA inhibition, increased neuronal firing, and phosphodiesterase (PDE) inhibition.10 Caffeine may also exert cholinergic effects via inhibition of acetylcholinesterase (Ki 175 μmol, equivalent to approximately 34 mg/L).10 In addition, caffeine can mobilize intracellular calcium, increase catecholamine levels, and increase the release and activity of excitatory amino acids and neurotransmitters.16,17,19 The increased heart rate in neonates given high doses of caffeine is due to increased adrenergic and dopaminergic effects.29
Gounaris et al. observed significantly delayed gastric emptying times during caffeine treatment in neonates with a birth weight of 1,000-1,500g and in those with a gestational age of 28-32 weeks.30 In a recent study, Huang X et al. showed that caffeine use was a risk factor for feeding intolerance in logistic multivariate regression analysis.31
Many infants continue to have apnea despite caffeine treatment, even with serum concentrations within the target range of 5-20 mg/L. This means that some infants may require plasma levels >15 mg/L to maximize the therapeutic effect of caffeine, providing some rationale for the use of higher doses to achieve therapeutic effect and clinical benefit. Several studies have evaluated higher versus lower doses of caffeine citrate using a variety of dosing regimens. Loading doses as high as 80 mg/kg and maintenance doses of up to 20 mg/kg/24h have been studied, with results indicating that the use of higher doses of caffeine may provide additional benefit in reducing the risk of bronchopulmonary dysplasia (BPD) compared to standard doses, but may increase the risk of cerebellar hemorrhage and seizures. Therefore, additional studies are needed before higher doses of caffeine can be routinely recommended. Increasing caffeine citrate by 1 mg/kg/24h every 1-2 weeks to a target dose of 8 mg/kg/24h may help maintain therapeutic effects.32 In 2017, Koch and colleagues developed simulation models of caffeine concentrations and suggested the need to adjust maintenance doses over time in preterm infants, with administration of 6 mg/kg/day in the second week of life, 7 mg/kg/day in weeks 3-4, and 8 mg/kg/day in weeks 5-8.33
Monitoring of plasma caffeine levels does not appear to be necessary with standard dosing regimens, but may be useful when higher than standard doses are used and in the presence of renal or hepatic impairment.10,34 The role of therapeutic drug monitoring needs to be addressed.35 It is recommended in neonates previously treated with theophylline or born to mothers who consumed caffeine prior to delivery.10
In adults, caffeine is extensively metabolized in the liver by CYP1A2 and CYP2E1 enzymes.10 In contrast, 85% of caffeine administered to the neonate is excreted unchanged in the urine.32 This explains the extremely slow plasma clearance and prolonged half-life of caffeine in preterm infants.11 The serum half-life of caffeine ranges from 40 to 230 hours and decreases with increasing postmenstrual age to 60 weeks. The half-life is prolonged in infants with cholestatic hepatitis.10 The development of caffeine tolerance represents another relevant gap in neonatal knowledge, as it may influence the selection of a standard dose regimen applicable to all neonates of different gestational and postnatal ages on prolonged caffeine therapy. It is unclear how long drug tolerance lasts after it is developed.10
The Caffeine for Apnea of Prematurity (CAP) trial
The CAP randomized controlled trial recruited preterm infants over five consecutive years (1999-2004) from 35 centers in Canada, Australia, Germany, Israel, the Netherlands, Sweden, Switzerland, the United Kingdom, and the United States. Preterm infants weighing 500-1,250 g were randomized to receive either placebo or caffeine citrate (20 mg/kg loading dose followed by 5 mg/kg/day maintenance, with a possible increase to 10 mg/kg/day) within the first ten days of life.7 The study drug was discontinued permanently at the discretion of local clinicians. However, it was recommended that the study drug be continued until the infant could tolerate at least five consecutive days without the use of positive airway pressure.7 In this study, 1,006 infants were randomized to caffeine and 1,000 were randomized to placebo. The median age at study entry was three days in both groups. The mean gestational age was 27 weeks in both groups, and the mean birth weight was 964 g in the caffeine group and 958 g in the placebo group. Compared to the placebo group, infants in the caffeine group were extubated and received their last positive pressure support at a median postmenstrual age of approximately one week earlier, and oxygen supplementation was stopped a median of 1.5 weeks earlier. Other short-term respiratory-related benefits of caffeine were lower rates of the following outcomes: medical treatment for patent ductus arteriosus (PDA) (caffeine 29%, placebo 38%), surgery for PDA (caffeine 4%, placebo 13%), post-randomization postnatal corticosteroids (caffeine 14%, placebo 20%), and bronchopulmonary dysplasia (BPD), defined as the need for supplemental oxygen at 36 weeks postmenstrual age (caffeine 36%, placebo 47%).7
Until 2006, only a few relatively small and short-term studies supported the use of caffeine. Thanks to the efforts of the CAP trial investigators, there are now high-quality, reliable data on not only short-term but also long-term outcomes of caffeine use in apnea of prematurity.
When to start and stop caffeine
Adenosine is a “defense” of the brain during ischemia.36 At therapeutic concentrations, methylxanthines are antagonists of adenosine receptors.36 Caffeine exposure has been associated with osteopenia of prematurity, transient increase in shunting across the ductus arteriosus, decreased flow in the superior mesenteric artery, and increased autonomic nervous system responsiveness associated with increased pulse pressure variability.37-40 Some authors have highlighted the very high dose administered to the smallest patients, equivalent to 10-14 cups of coffee.41 It has been suggested that the addition of this drug to the milieu of catecholamines, corticosteroids, and thyroid hormones in the preterm infant may not be benign. Several studies have addressed the issue of early versus late initiation of caffeine therapy, and based on the currently available evidence, early routine caffeine prescription does not appear to be indicated.42 Controversy remains regarding the optimal timing and dosage of caffeine therapy.35 Caffeine initiation is indicated for infants with apnea and for those who are about to be extubated, and clinicians can prescribe the drug with confidence using standard doses.43 The clinical benefits of initiating caffeine therapy before three days of age have been recently summarized by Dobson and Hunt, who showed that early treatment reduces the incidence of BPD (with moderate quality of evidence according to the Grading of Recommendations Assessment, Development and Evaluation system), death or BPD, intraventricular hemorrhage (IVH), necrotizing enterocolitis, need for PDA treatment, retinopathy of prematurity, and use of postnatal steroids (all with low quality of evidence) .44
Regarding caffeine discontinuation, the CAP trial encouraged local clinicians to stop the study medication when they felt it was no longer needed.7 However, it recommended that therapy be continued until the infant could be managed without any positive pressure support, including supplemental oxygen, for at least five consecutive days. Infants in the control group discontinued study medication at a median interquartile range of 34.7 (32.9-36.1) weeks postmenstrual age, and those randomized to caffeine discontinued study medication at 34.4 (33.0-35.9) weeks postmenstrual age. There is little high-quality evidence that caffeine is a safe and effective “brain” or “lung” drug, and its use in asymptomatic infants who do not require respiratory support does not appear warranted, regardless of postmenstrual age.41 A reasonable approach is to start caffeine when apnea develops in infants >28 weeks who do not require invasive respiratory support. Earlier (<3 days) versus later ( >3 days) prophylactic caffeine has been studied in infants requiring mechanical ventilation, but the safety and efficacy of this approach require further investigation.45
Effects of caffeine on the risk of bronchopulmonary dysplasia
The CAP trial was conducted to evaluate the short- and long-term efficacy and safety of caffeine therapy in very low birth weight neonates.7,43 The trial showed that, compared with placebo, caffeine significantly reduced the incidence of BPD, as defined by the need for supplemental oxygen at 36 weeks postmenstrual age.7 Among infants alive at 36 weeks, 36.3% (350/963) in the caffeine group and 46.9% (447/954) in the placebo group developed BPD.7 After adjustment for study center, caffeine reduced the odds of BPD by 37% (adjusted odds ratio 0.63, 95% CI 0.52-0.76).7 Using the BPD rate of 46.9% in the placebo group to define the baseline risk of BPD, the average number of infants who would need to be treated with caffeine to prevent one case of BPD was 10 (95% CI 7, 16).46 This study identified other benefits of caffeine, including reduced frequency of postnatal corticosteroid therapy, reduced rates of need for treatment of patent ductus arteriosus (PDA), and reduced severity of retinopathy of prematurity.7,43 A very important finding of the CAP trial was that the results for the primary outcome showed significantly improved survival rates without neurodevelopmental disability at 18-21 months corrected age in caffeine-treated infants.43 Follow-up of study participants up to 11 years of age showed that caffeine produced sustained improvements in pulmonary mechanics and motor function in these infants.47,48
Approximately one-third of the CAP trial participants who were assigned to receive caffeine therapy developed BPD.7 An important question is whether caffeine reduces the severity of BPD in infants who ultimately develop the disease. To date, no randomized controlled trial has provided robust evidence to answer this specific question. However, the CAP trial showed that compared with placebo, caffeine resulted in earlier successful extubation (from a median PMA of 30.0 to 29.1 weeks), discontinuation of positive airway pressure (from 32.0 to 31.0 weeks), and discontinuation of oxygen therapy (from 35.1 to 33.6 weeks).7 Meta-analyses of other older and smaller trials have shown that methylxanthine results in less frequent initiation of mechanical ventilation and lower risk of extubation failure.49,50 Finally, physiologic studies suggest that caffeine improves minute ventilation, reduces total lung resistance, and increases lung compliance in ventilator-dependent preterm infants with BPD.51 These data collectively support the hypothesis that caffeine therapy initiated in the early neonatal period may lead to less severe BPD in infants who develop the disease. However, the true effects of caffeine on the severity of BPD remain uncertain.
The CAP trial demonstrated that caffeine, initiated within the first ten days of life, is one of the few drug therapies that significantly reduces the risk of BPD in very low birth weight infants. This beneficial effect is (at least in part) likely due to reduced exposure to positive airway pressure and supplemental oxygen, two key risk factors for BPD. Additional cardiorespiratory benefits of caffeine that may contribute to the reduced risk of BPD include less frequent treatment for PDA, improved pulmonary mechanics, and direct effects on pulmonary inflammation, alveolarization, and angiogenesis.52
Caffeine and ductus arteriosus
The CAP trial showed a reduction in the use of both pharmacologic and surgical treatment of PDA in the group of infants receiving caffeine therapy.7 It is not clear whether this beneficial effect is due to a direct effect of caffeine on the ductus arteriosus or other mechanisms. Caffeine has been shown to directly affect several of the signaling molecules involved in constriction of the ductus arteriosus. Caffeine increases cAMP by downregulating cyclic nucleotide phosphodiesterase activity and inhibits prostaglandin production and activity.53,54 However, a study in fetal lambs found no direct effect of caffeine on ductal contractility.55 Another study in preterm infants observed increased rather than decreased flow through the ductus following administration of a caffeine loading dose.38 Caffeine may promote greater clinical stability by reducing apnea, increasing diuresis, and allowing earlier weaning from respiratory support.7,50,56 As a result, clinicians may become less aggressive in diagnosing and treating PDA.
Long-term respiratory outcomes
Doyle et al. measured expiratory flow rates according to American Thoracic Society standards in children enrolled in the CAP trial at age 11 years.57 Values were converted to z-scores predicted by age, height, ethnicity, and sex, and parents completed questionnaires about their child's respiratory health. A total of 142 children were studied, with results showing improved expiratory flows in the caffeine group - approximately 0.5 standard deviation [SD] for most variables (e.g., FEV1; mean z-score, -1.00 vs. -1.53; mean difference, 0.54; 95% confidence interval [CI], 0.14-0.94; P=0.008). Fewer children in the caffeine group had forced vital capacity (FVC) below the fifth centile (11% vs. 28%; odds ratio, 0.31; 95% CI, 0.12-0.77; P=0.012). After adjustment for BPD, the difference in flow rates between groups was reduced. The authors concluded that caffeine treatment in the neonatal period improves expiratory flow rates in mid-childhood, which appears to be achieved by improving respiratory status early in life.57
At age 11 years, fewer children in the caffeine group had received wheezing medication in the previous 12 months, and other symptoms related to asthma were less common than in the placebo group, although there were no substantial differences between both groups. A lifetime diagnosis of asthma was less common in the caffeine group, but again there was little evidence of a substantial difference between groups. Hospital readmissions for respiratory problems in the previous 12 months were uncommon in both groups.53 This study by Doyle LW et al. showed that the short-term benefits of caffeine on the lungs of newborns translated into better lung function and respiratory health later in childhood.57
Neurodevelopmental outcomes following caffeine therapy
Neurodevelopmental disability in preterm infants is primarily due to acquired brain injuries such as IVH and periventricular leukomalacia (PVL). Brain magnetic resonance imaging (MRI) has provided additional data on the importance of white matter injury. Young oligodendroglia are susceptible to hypoxia/ischemia and inflammation, which do not affect mature myelin-forming oligodendrocytes.58 Impaired maturation, whereby pre-oligodendrocytes fail to differentiate (also known as dysmaturation), is the primary mechanism of myelination failure in preterm infants.58 Dysmaturation, with resulting white matter injury, is typically diffuse and is the most important predictor of neurodevelopmental impairment in preterm infants.58 Gray matter is also affected, and the most vulnerable regions are the subcortical basal ganglia, thalamus, hippocampus, cerebellum, and cortical regions.58
Few neuroprotective strategies have been identified, and interest in the neuroprotective potential of caffeine has arisen after findings that caffeine used to treat or prevent apnea of prematurity improved survival without neurodevelopmental disability at 18-21 months corrected age in infants in the CAP trial.43 Survival with one or more major impairments (cerebral palsy, cognitive delay [Mental Development Index < 20/200]) was the primary neurodevelopmental outcome assessed at 18 months corrected age in these children. Adequate outcome data were available for 1869 infants (93.2%). No significant differences were found in deafness and bilateral blindness between the two groups, but the rate of these disabilities was low. The incidence of cerebral palsy (4.4% vs. 7.3%) and cognitive delay (33.8% vs. 38.3%) was significantly lower in the caffeine group. Therefore, at 18-21 months corrected age, caffeine significantly improved survival without neurodevelopmental disability.43
At five years of age, the goal was to determine whether neonatal caffeine therapy had lasting benefits or new apparent risks at the age of school entry.59 The primary outcome was a composite of death before five years or survival with one or more of the following disabilities: motor impairment (Gross Motor Function Classification System level 3 to 5), cognitive impairment (Full Scale IQ <70 on the Wechsler Preschool and Primary Scale of Intelligence 3rd edition), behavior problems, poor general health, severe hearing loss, or bilateral blindness. A behavior problem was defined as a Total Problem T score >69 (2 SD above the mean) on the Child Behavior Check List (CBCL). Poor general health included several ongoing medical morbidities, such as the need for supplemental oxygen, tube or intravenous feeding, or seizures. Severe hearing loss was defined as the need for hearing aids or cochlear implants, and bilateral blindness was defined as corrected visual acuity (CI, 0.47-0.88). Similarly, the caffeine group had better mean scores for manual dexterity, visual perception, and fine motor coordination. In addition, in a post hoc analysis, Doyle, Schmidt, and colleagues found a reduction in developmental coordination disorder with neonatal caffeine therapy.60 In contrast, mean scores did not differ for cognitive outcomes or for the CBCL Total Behavior Problems. For the 11-year follow-up, 13 sites in Canada, Australia, the United Kingdom, and Sweden participated, with data available for 870 children (n=457 in the caffeine group and n=463 in the placebo group).61 The primary outcome was functional impairment, a composite of academic performance, motor function, and behavior. The motor impairment component significantly favored the caffeine group. Secondary outcomes were measures of general intelligence (Wechsler Abbreviated Scale of Intelligence-II), attention (Test of Everyday Attention for Children), executive function (Rey Complex Figure [RCF] test), visual motor perception, visual motor integration (VMI), and behavior (Conners 3 Attention-Deficit Hyperactivity Disorder Index; Behavior Rating Inventory of Executive Function). At the 11-year follow-up, the groups remained comparable for age, school attendance, and family characteristics. Caffeine significantly reduced the risk of motor disability, and significant benefits of caffeine were seen for visual perception, fine motor coordination, and RCF copying accuracy. No statistically significant differences were found for cognitive measures, attention, other executive functions, and parent-rated behavioral questionnaires.61,62) In summary, caffeine treatment reduced the rate of cerebral palsy and cognitive impairment at 18 months and showed consistent benefits for motor development at 18 months, five years, and 11 years in the CAP trial. Gross and fine motor skills were associated with neonatal caffeine therapy at school entry and in middle childhood, with better visual-motor, visuospatial, and fine motor skills. Importantly, no adverse effects on behavior, intelligence, attention, or executive function were observed.63
Neuroimaging
Although no between-group differences in cranial ultrasound abnormalities were found in the CAP trial, a subset of 70 preterm infants in Australia were enrolled in a study of brain MRI at term-equivalent age.64 No differences were found in the extent of white or gray matter abnormalities or in brain volume, but 28 infants with diffusion tensor imaging showed that caffeine treatment was associated with better white matter microstructural development, with the greatest effects in the superior brain regions. These changes were independent of BPD.65 However, no differences in brain volume and white matter were observed on brain MRI between the caffeine and placebo groups at 11 years of age.66 The caffeine group had slower growth of the corpus callosum. Therefore, there is no evidence for long-term benefits of caffeine on brain imaging.
Retinopathy of prematurity
The CAP trial demonstrated a reduction in retinopathy of prematurity.7 Retinopathy of prematurity, BPD, and abnormal brain imaging are significant predictors of adverse neurodevelopmental outcomes.67
Growth and metabolism
Caffeine increases energy expenditure and oxygen consumption in the body and is associated with less weight gain and lower incubator temperature, with no difference by dose.67 In the CAP trial, the caffeine group had lower weight gain in the first three weeks of life, but no differences were observed in growth at 18 months corrected age.7
The daily and cumulative doses of caffeine and smaller gestational age were shown to be associated with the development of osteopenia of prematurity in a study by Kumar et al. The authors concluded that randomized controlled trials are needed to validate the outcomes and determine the range of the safest and most effective caffeine doses for the treatment of apnea of prematurity in preterm infants.68
Conclusions
Caffeine, a methylxanthine inhibitor of adenosine receptors, has been used for more than four decades to treat or prevent apnea of prematurity and is one of the most commonly prescribed drugs in neonatal medicine.
The standard and commonly prescribed doses of caffeine citrate (20 mg/kg loading and 5-10 mg/kg/day maintenance) exert their pharmacodynamic effects on respiratory control and reduce apnea of prematurity. At higher doses, caffeine may interact with multiple molecular targets and produce adverse pharmacologic effects.
The optimal timing and dosage remain controversial. For infants with apnea and those about to be extubated, initiation of caffeine is indicated, and clinicians can prescribe the drug with confidence using standard doses. A reasonable approach is to start caffeine when apnea develops in infants >28 weeks who do not require respiratory support, and early (less than three days) prophylactic caffeine in infants who do require respiratory support. Monitoring of plasma caffeine levels does not appear to be necessary with standard dosing regimens, except in neonates previously treated with theophylline or born to mothers who consumed caffeine before delivery and in infants with hepatic and renal impairment.
Clinicians can stop caffeine when they feel it is no longer needed.
Other benefits of caffeine treatment have been demonstrated and include a reduction in the duration of positive pressure support and oxygen supplementation, the use of postnatal corticosteroid therapy and BPD, lower rates of need for treatment of PDA, a reduction in the severity of retinopathy of prematurity, improved rates of survival without neurodevelopmental disability at 18-21 months corrected age, and sustained improvements in pulmonary mechanics and motor function at 11 years of age. Despite the large number of studies on the benefits of caffeine, there is a paucity of data on its effects on the gastrointestinal tract of very preterm infants and, in particular, on gastric emptying time.