Introduction
Extremely preterm infants are at significant risk for low systemic blood flow (SBF) and inadequate organ perfusion. Low SBF has been documented in up to 35% of infants born less than 30 weeks gestational age (GA).1 It typically develops within the first 12 hours of life, followed by improvement over 24-48 hours, and is associated with adverse neurological outcomes.2,3
At birth, infants undergo a series of complex adaptive sequences from fetal to postnatal circulation. Distinguishing physiological transition from pathological changes in neonatal cardiovascular status is often problematic due to the lack of consensus surrogates for low perfusion. When low SBF is suspected, it can also be difficult to determine the underlying pathophysiologic process and decide on appropriate pharmacologic management. These challenges are reflected in the significant variability in the assessment and management of perfusion states across countries and centers.4
Objectives
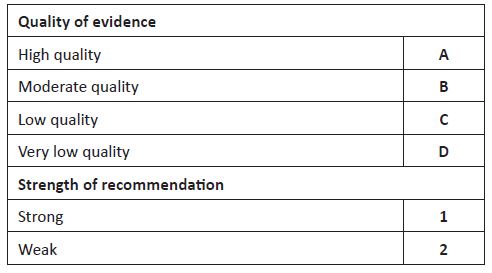
The aim of this article was to review the methods used in clinical practice for assessment of low SBF and present a pathophysiology-based approach to the management of low blood flow states in extremely preterm infants. A search was performed on Medline and evidence up to February 2022 was reviewed. Quality of evidence and strength of recommendations were appraised as described in Table 1.
BP, Blood Pressure; CO, Cardiac Output; CRT, Capillary Refill Time; EPT, Extreme Preterm; FO, Foramen Ovale; hsPDA, Hemodynamically Significant Patent Ductus Arteriosus; iNO, Inhaled Nitric Oxide; IV, Intravenous; LV, Left Ventricle; MAP, Mean Airway Pressure; NEC, Necrotizing Enterocolitis; NIRS, Near Infrared Spectroscopy; PRBC, Packed Red Blood Cells; rScO2, Regional Cerebral Oxygen Saturation; SBF, Systemic Blood Flow; SVC - Superior Vena Cava.
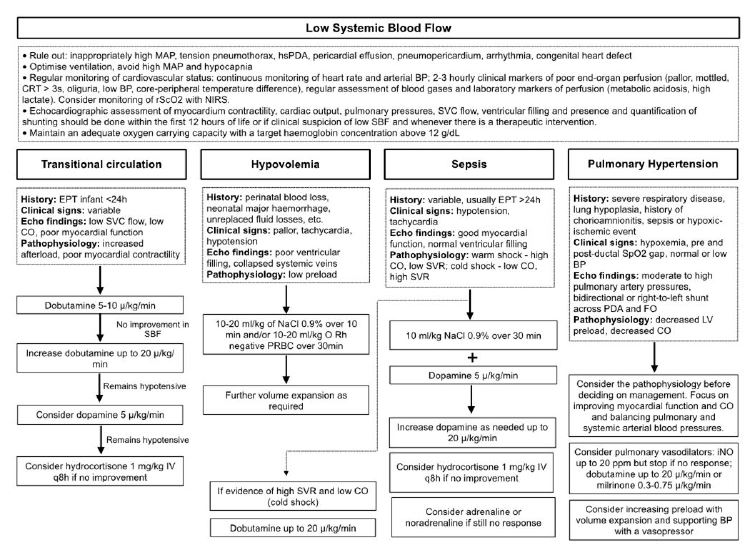
Figure 1 Algorithm for pathophysiology-based management of low systemic blood flow in extremely preterm infants (adapted from “Hemodynamically based pharmacological management of circulatory compromise in the newborn” in Seri, I., Kluckow, M. (3rd ed.) Neonatology Questions and Controversies - Hemodynamics and Cardiology)
Table 2 Dose and mechanism of action of drugs used in the treatment of infants with cardiovascular compromise
| Dose | Effect | Considerations | |
| Dopamine | 2-20 µg/kg/min | Dopaminergic receptor: increases renal blood flow (at low dose). Cardiac β-1 and β-2: positive inotropy (contractility) and chronotropy (heart rate) (at intermediate dose). Peripheral α-1 and α-2: increases SVR (at high dose). | The dose-dependent effect varies widely among neonates. Increases mean arterial pressure primarily through vasoconstriction. At high doses, the increase in SVR may impair contractility. When used in PPHN, the increase in PVR may compromise oxygenation. |
| Dobutamine | 5-25 µg/kg/min | Cardiac β-1 and β-2: positive inotropy and chronotropy (low and high doses). Peripheral β-2: decreases SVR (high doses). | Increases cardiac output but not mean arterial pressure. |
| Adrenaline | 0.01-1 µg/kg/min | Cardiac β-1 and β-2: inotropic effect (low doses). Peripheral α-1 and α-2: increases SVR (high doses). | Increases SVR to a greater extent than PVR. May increase plasma lactate and glucose. |
| Noradrenaline | 0.2-2 µg/kg/min | Predominantly peripheral α-1 and α-2: increases SVR. | Increases SVR to a greater extent than PVR. Increases peripheral ischemia and acidosis at doses >0.33 µg/kg/min. |
| Vasopressin | 0.0001-0.002 units/kg/min | V1: increases SVR and decreases PVR. | Has no effect on contractility. |
| Milrinone | 0.3-0.75 µg/kg/min | Phosphodiesterase III inhibitor that prevents cAMP breakdown - inotropic, lusitropic (improves myocardial relaxation), and vasodilator effect. | Reduces afterload by decreasing SVR. May cause hypotension. Avoid loading dose in preterm infants. |
| Hydrocortisone | 1 mg/kg IV q8h | Increases the sensitivity of adrenergic receptors to catecholamines, increases their expression, and reduces the local production of vasodilators. | Increases blood pressure and decreases vasopressor requirements. |
| iNO | 20 ppm | Stimulates guanylate cyclase enzyme to produce cGMP. | Selective pulmonary vasodilator that is effective in PPHN. |
cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; iNO, inhaled nitric oxide; IV, intravenous; PPHN, persistent pulmonary hypertension of the newborn; ppm, part per million; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance; V1, vasopressin V1 receptor.
Etiology of low blood flow states in extremely preterm infants
The pathophysiology of low SBF differs between early and late circulatory compromise. In extremely preterm infants within the first 24 hours of life, low SBF is often related to transitional circulation, whereas later circulatory compromise is often due to sepsis, necrotizing enterocolitis, or high-volume shunting through a patent ductus arteriosus (PDA).
The multitude of cardiorespiratory changes that occur during the transition to extrauterine life are driven by two events: lung aeration and umbilical cord clamping. In utero, pulmonary vascular resistance (PVR) is high and pulmonary blood flow is low, with much of the systemic flow dependent on right ventricular output through the ductus arteriosus (DA). After birth and adequate lung aeration, PVR progressively decreases, leading to an increase in pulmonary venous return and left atrial preload. With cord clamping, there is a loss of placental low vascular resistance with an increase in systemic vascular resistance (SVR) and afterload. The increase in SVR and decrease in PVR results in a change in the direction of blood shunting across the DA and patent foramen ovale from left to right. In preterm infants, this physiological transition is influenced by several factors, including organ system immaturity, maternal conditions and medications, timing of umbilical cord clamping, and resuscitation maneuvers.5 In this context, circulatory compromise may be multifactorial in origin: decreased preload (especially with immediate cord clamping, peripartum blood loss, or sepsis-related capillary leak); impaired contractility of the immature myocardium in response to increased afterload; inappropriately high airway pressure that compromise venous return and cardiac output; persistence of fetal shunts that divert blood away from the systemic circulation; and changes in peripheral vascular resistance secondary to sepsis.6
Compared to their term counterparts, preterm infants have impaired systolic and diastolic function. The preterm myocardium is more sensitive to increases in afterload and responds with ineffective contractility.7 Diastolic function is impaired due to high levels of noncontractile and noncompliant collagen fibers, which condition abnormal relaxation.7 This is compounded by the increased heart rate in neonates compared to older children, meaning that less time is spent in diastole.8 In addition, there is often higher systemic vascular tone due to an increased alpha to beta receptor ratio.8
In the presence of a hemodynamically significant PDA, in addition to the “ductal steel phenomenon”, where there is a high volume of shunting from the systemic to the pulmonary circulation, the systolic performance of the preterm myocardium may fail to adapt appropriately to the increased pulmonary venous return.8 Ultimately, the left ventricle may be unable to maintain stroke volume according to the Frank-Starling relationship, leading to pulmonary congestion, systemic hypoperfusion, and severe end-organ compromise.8
Monitoring of extremely preterm infants
a) Clinical and laboratory markers
Clinical and laboratory markers have been shown to be largely unreliable in detecting low SBF. Since blood pressure (BP) is the dependent variable in the equation BP = Cardiac Output x Resistance, it follows that hypotension can occur without a change in cardiac output, and a decrease in cardiac output can occur with normal BP if vascular resistance is increased. However, hypotension remains the most commonly used marker of low perfusion in clinical practice, occurring in up to 20% of very low birth weight (VLBW) infants.9 Despite an association between hypotension and poor short-term outcomes, such as intraventricular hemorrhage (IVH), studies using echocardiographic markers have found a poor correlation between hypotension and low SBF.1,10-12 In addition to uncertainty about the role of BP in assessing SBF, there is considerable controversy over the definition of hypotension. Most clinicians define hypotension as a mean arterial blood pressure (MABP) in mmHg less than the GA in weeks, which roughly correlates with the 10th centile of normative reference ranges for the first few days of life.4,13 Another commonly used definition is a MABP below 30 mmHg, based on the association of this threshold with reduced cerebral blood flow and cerebral injury.14,15 While the 10th centile of MABP may be below 30 mmHg in the first two to three days of life, all infants, regardless of GA, are expected to have a MAPB above this threshold.13 However, these definitions are a simplification of a complex problem. The critical BP threshold is the minimal BP at which organ perfusion is compromised, which is likely to be specific to an individual patient and influenced by multiple factors.16 Furthermore, relying solely on MABP rather than evaluating the systolic and diastolic components according to normative data may neglect important insights into the pathophysiology behind low BP. Systolic BP is considered a surrogate for left ventricular stroke output, whereas diastolic BP reflects SVR.7
Other parameters used in clinical practice, including capillary refill time (CRT), core-peripheral temperature difference, urine output, and serum lactate, have poor individual correlation with SBF, but their combination appears to improve their predictive value. Milletin et al. found that the combination of CRT > 4 seconds in the foot and lactate > 4 mmol/L resulted in a positive predictive value of 80% and a negative predictive value of 88% for low flow states in a group of VLBW infants.17 A recent retrospective analysis found that most clinicians used MABP GA minus 1 mmHg and lactate > 4 mmol/L as biomarkers for initiating cardiovascular treatment.18
b) Echocardiographic markers
In adult and pediatric patients, SBF is typically estimated from echocardiographic measurements of left and right ventricular output, but in preterm infants these can be affected by left-to-right shunting across the DA and patent foramen ovale, which can be significant within the first few postnatal hours.19 In the presence of a PDA, left ventricular output will overestimate SBF because some of the output is shunted across the ductus into the pulmonary circulation. The increase in left atrial pressure as a result of the increase in pulmonary venous return will cause a left to right shunt across the foramen ovale, which in turn may lead to an overestimation of SBF when measuring right ventricular output as a representation of SBF. Kluckow and Evans proposed Doppler assessment of superior vena cava (SVC) flow as a proxy for SBF.2 SVC flow represents blood coming from the upper body, with an estimated 80% coming from the head, and as such SVC flow can also be used as a general measure of cerebral perfusion.20 Low SVC flow has been associated with IVH, neurodevelopmental disability at three years, and death.1,21,22
Evaluation of myocardial contractility and volume status (ventricular filling, collapsed systemic veins) by functional echocardiography (fECHO) complements the cardiovascular assessment, with serial measurements assisting in the evaluation of treatment response. Left ventricular systolic performance can be assessed using shortening fraction (SF; normal 28-40%), ejection fraction, or rate-corrected mean velocity of circumferential fiber shortening.23 SF must be interpreted with caution in the first days of life when high right ventricular pressure may impair ventricular septal motion.23 fECHO should also be used to determine the presence, direction, and magnitude of shunting through the ductus arteriosus.
c) Continuous monitoring of microcirculation and organ blood flow
In infants with cardiovascular compromise, peripheral vasoconstriction may occur in an attempt to redirect blood to vital organs, suggesting that microcirculatory monitoring could lead to early detection of hypoperfusion.24 The perfusion index (PI) is a numeric value derived from some pulse oximeters that allows assessment of peripheral perfusion. It is extrapolated from the ratio of the pulsatile signal, determined by arterial blood flow, to the absent pulsatile signal, determined by skin, subcutaneous tissue, venous blood flow, and other local tissues.24 In the presence of local vasoconstriction, the PI value decreases, with PI values below 0.44 correlating with low SVC flow.24,25 Decreases in PI may reflect the physiologic variability in peripheral microvascular blood flow that characterizes the transitional period, but may also correlate with low cardiac output and predict mortality and morbidity in extremely preterm infants.26,27
The use of near-infrared spectroscopy (NIRS), a non-invasive technique, to assess target organ blood flow in newborn infants has increased in recent years. NIRS provides a continuous measurement of tissue hemoglobin oxygen saturation, reflecting a mixed oxygen saturation of arterial, capillary, and venous perfusion dominated by the venous component.28 Cerebral fractional tissue oxygen extraction (cFTOE) can be calculated from regional cerebral oxygen saturation (rScO2) and arterial oxygen saturation (SaO2).28 An elevated cFTOE indicates decreased blood supply or increased oxygen consumption.28 Clinical studies suggest that NIRS is a useful adjunct tool in the identification and management of preterm infants with cardiovascular compromise. Observational studies have shown that lower rSCO2 in the delivery room predicts the development of IVH in preterm infants.29 Preterm infants with PDA had lower rSCO2 and higher cFTOE compared to controls, whereas closure of PDA was associated with an increase in rSCO2 and a decrease in cFTOE.30,31 In the SafeBoosc II trial, extremely preterm infants randomized to rScO2 monitoring during the first 72 hours of life had a lower median burden of hypoxia and hyperoxia compared to blinded rScO2 monitoring controls.32 Ongoing large randomized controlled trials (RCTs) hypothesizing that NIRS monitoring reduces the risk of poor short-term outcomes by allowing early identification and subsequent treatment of infants at risk will provide relevant data to understand the clinical usefulness of NIRS.33,34
Prevention of low systemic blood flow
Perinatal interventions known to improve short- and long-term outcomes may also improve transitional circulation hemodynamics. Antenatal steroids decrease the risk of hypotension and low SVC flow and increase the rate of response to inotropes in preterm infants.35,36 Although some evidence suggests that delayed cord clamping decreases the risk of hypotension, a recent large RCT showed that cord clamping for 60 seconds or longer had no effect on SVC flow in the first 24 hours of life in infants less than 30 weeks GA compared with cord clamping for less than 10 seconds.37,38 Whether physiologic cord clamping or lung aeration with positive pressure ventilation while still attached to the placenta improves SBF in these infants remains to be determined. A double-blind RCT evaluating whether the vasodilator and inotropic effects of prophylactic milrinone initiated within the first six hours of life could prevent low SBF in infants less than 30 weeks GA found no significant difference in SVC flow, right ventricular output, or BP between infants treated with milrinone and those treated with placebo.39
Management of low systemic blood flow
The goal of pharmacologic management of low SBF is to ensure adequate perfusion and oxygen delivery. A pathophysiology-based approach is recommended (Figure 1). A summary of the effects of commonly used drugs in infants with circulatory compromise is presented in Table 2.
a) General management
Early recognition and treatment of low SBF is essential to preserve organ perfusion and function. Perinatal/neonatal history, clinical and laboratory markers of perfusion, NIRS, and echocardiography should be used to diagnose low SBF, determine its pathophysiology, and guide management. General management of extremely preterm infants should include:
Regular monitoring of hemodynamic status (especially during the first 24 hours of life), including heart rate, arterial BP, and clinical markers of poor end-organ perfusion (pallor, mottled, CRT over three seconds, oliguria, low BP, core-peripheral temperature difference), and assessment of laboratory markers of perfusion (metabolic acidosis, high lactate). Although invasive arterial BP monitoring is not required in all preterm infants, it is important to note that noninvasive BP measurements often overestimate true arterial BP. Infants with low BP and signs of poor perfusion in the first 24 hours of life require medical intervention. Current international expert recommendations are that a watchful waiting approach can be adopted for infants with isolated hypotension, based on evidence that untreated hypotensive infants without other signs of poor perfusion have similar outcomes to normotensive infants, suggesting that a “permissive hypotension” approach can be safely implemented.40,41 However, results from the French EPICAGE 2 study have raised concerns about this approach.42 In this matched cohort study, the authors compared extremely preterm infants with isolated hypotension who received treatment with untreated hypotensive infants during the first three days of life. They found that antihypotensive treatment was associated with a significantly higher survival rate without major morbidity at discharge and a lower incidence of severe cerebral lesions, particularly in infants with a minimum MABP of GA (5 mmHg).
Echocardiographic assessment of myocardial contractility, cardiac output, pulmonary pressure, SVC flow, and ventricular filling and quantification of shunting within the first 12 hours of life or when low SBF is clinically suspected and at any time of therapeutic intervention.
Ventilation optimization - High mean airway pressure may impede venous return and reduce preload; hypocapnia may exacerbate low cerebral blood flow and increase the risk of brain injury, possibly through a mechanism of hypoperfusion.16
Maintenance of adequate oxygen-carrying capacity with a target hemoglobin concentration above 12 g/dL.40
b) Pharmacological management
Volume expansion
Volume expansion is administered to increase preload in infants with cardiovascular compromise. It is essential in hypovolemia, but its effect in normovolemic infants with low SBF is uncertain. Evidence supporting the use of volume expansion is inconsistent. Some studies have found that volume expansion improves BP, while others have found no change.36,43-45 Most studies have found that volume expansion does not improve cerebral blood flow indices.43-45 Excessive volume expansion over 30 mL/kg has been associated with increased mortality in preterm infants, and in a recent retrospective cohort study of infants less than 34 weeks GA and/or 1500 g, the authors found that infants who received at least one 10 mL/kg fluid bolus within the first 48 hours had a higher prevalence of PDA and IVH and were more likely to require home oxygen.46,47
Recommendation (B1): Volume expansion should be given as needed in hypovolemic infants. There is some evidence that volume expansion may be detrimental in normovolemic infants with cardiovascular compromise. Therefore, the authors discourage the use of fluid boluses in preterm infants, particularly during the first two to three days of life, and suggest that they be reserved for infants with echocardiographic evidence of poor filling status.
Dopamine
Dopamine is an endogenous catecholamine precursor of noradrenaline. It has a dose-dependent effect on dopaminergic, β- and α-adrenoceptors. Dopamine has been shown to be effective in raising BP through its inotropic and vasopressor actions, but with conflicting evidence regarding its effects on SBF.48 Some studies have found that dopamine did not improve left ventricular output or SCV flow and either showed no effect or worsened cerebral and mesenteric perfusion indices in hypotensive infants, whereas other studies have shown an improvement in left ventricular output, cerebral blood volume, and cerebral intravascular oxygenation.36,44,49-51 A meta-analysis of mostly observational studies found an improvement in cerebral blood flow after dopamine administration in hypotensive preterm infants.53 The Hypotension in Preterm Infants (HIP) trial, a double-blind, placebo-controlled, randomized trial that was terminated early due to enrollment issues, randomized hypotensive extremely preterm infants to dopamine or placebo infusion.54 The trial found no difference in survival to 36 weeks postmenstrual age without severe brain injury between groups, but infants in the control arm were more likely to require additional treatment for low BP. A recently published prospective cohort study within the HIP trial found that dopamine did not improve cerebral oxygenation in hypotensive extremely preterm infants compared with placebo.55
Recommendation (C2): The vasopressor effect of dopamine is effective in raising BP, but may further compromise SBF in extremely preterm infants with myocardial dysfunction and high postnatal afterload. In sepsis, where hypotension is largely due to peripheral vasodilation, a vasopressor is recommended and dopamine, which is widely studied and used, is preferred.
Dobutamine
Dobutamine is a synthetic catecholamine with predominantly β-adrenergic effects. Compared with dopamine, dobutamine has been shown to be less effective in improving BP in hypotensive infants, probably because of its vasodilatory effects. On the other hand, dobutamine is more consistent in improving echocardiographic markers of SBF. A Cochrane meta-analysis found that while dopamine had a greater effect on BP, dobutamine was associated with a greater increase in left ventricular output in hypotensive preterm infants.48 Similarly, a RCT found that dobutamine resulted in greater improvement in SVC flow and right ventricular output compared with dopamine.36 A follow-up of this trial found no difference in the combined rate of death and neurodevelopmental disability at three years.22 One of the few placebo-controlled RCTs available found that infants treated with dobutamine showed a trend toward earlier normalization of SVC flow and a statistically significant improvement in markers of perfusion compared with placebo.56 Interestingly, SVC flow improved in both the placebo and dobutamine groups, suggesting that SBF improves naturally over time regardless of intervention. A follow-up of this trial showed no difference between groups in long-term neurodevelopmental outcomes.57
Recommendation (C1): The effects of dobutamine on myocardial contractility and systemic vascular resistance make it a first-line agent when myocardial dysfunction and increased afterload are the primary determinants of low SBF, as in the transitional circulation of extremely preterm infants in the first 24 hours of life.
Adrenaline and noradrenaline
Adrenaline is an endogenous catecholamine with predominantly β-adrenergic effects at lower doses and α-adrenergic effects at higher doses. Noradrenaline is a sympathomimetic amine with predominantly α-agonist effects. Few studies have investigated the use of these drugs in newborn infants. A blinded RCT comparing dopamine and adrenaline in hypotensive preterm infants under 32 weeks GA found no difference in BP or cerebral blood flow indices, but an increased incidence of high lactate and hyperglycemia in the adrenaline-treated group.52 A follow-up study evaluated neurodevelopmental outcomes at two to three years and found no difference between the two groups.58 A retrospective study of preterm infants treated with noradrenaline, mostly for septic shock or pulmonary hypertension, found that it was well tolerated and effective in increasing BP.59 A prospective study evaluated the effects of noradrenaline in infants over 35 weeks’ gestation with septic shock refractory to volume expansion and dopamine/dobutamine and found that noradrenaline improved BP and other markers of perfusion, such as urine output and lactate.60
Recommendation (D2): Adrenaline and noradrenaline have vasoconstrictive effects that may be beneficial in vasodilatory shock. Due to the lack of data, these agents should be used as second-line agents in dopamine-refractory shock.
Vasopressin
Vasopressin is a naturally occurring vasoconstrictive hormone. In observational studies of infants with inotrope-refractory hypotension, vasopressin appeared to be effective in raising BP and improving urine output.61,62 Compared with dopamine, vasopressin was found to be equally effective in raising BP with less tachycardia in the treatment of hypotensive preterm infants within the first 24 hours of life.63 In a recent case series, Mohamed et al. found that vasopressin improved oxygenation and other hemodynamic variables in preterm infants with persistent nitric oxide-refractory pulmonary hypertension of the newborn.64
Recommendation (D2): Vasopressin may have a role in inotrope-refractory vasodilatory shock and persistent pulmonary hypertension of the newborn, but limited evidence of efficacy and safety precludes its routine use.
Milrinone
Milrinone is a selective phosphodiesterase type III inhibitor that decreases vascular tone and increases myocardial contractility. Despite evidence suggesting that prophylactic milrinone does not prevent low SBF, whether it is beneficial in the treatment of low SBF in preterm infants remains unknown.39 There is some evidence that milrinone may be beneficial in low SBF after PDA ligation and in persistent pulmonary hypertension of the newborn.65,66
Recommendation (D2): Milrinone reduces pulmonary and systemic vascular resistance and may have a role in the management of persistent pulmonary hypertension of the newborn and high afterload, but a lack of data precludes its routine use in preterm infants.
Hydrocortisone
Due to immature adrenal function, preterm infants may develop relative adrenal insufficiency. Corticosteroids act by increasing receptor sensitivity to catecholamines, increasing their expression, and decreasing local production of vasodilators. Low cortisol levels are associated with low BP, suggesting a role for this drug in the management of hypotension.67 Prophylactic hydrocortisone started within the first three hours of life appears to reduce the incidence of hypotension in extremely low birth weight infants compared with placebo.68 Compared with dopamine, hydrocortisone has been found to be less effective in raising BP in hypotensive preterm infants.69 In a double-blind, placebo-controlled RCT, Ng et al. found that the addition of hydrocortisone improved BP and allowed faster weaning from inotropes in VLBW infants with dopamine-refractory hypotension.70 These effects were confirmed in a prospective observational study by Noori et al. in preterm and term infants with dopamine-refractory hypotension.71 The authors also found that hydrocortisone did not affect cardiac output or systemic perfusion. A Cochrane review cautioned against routine administration of hydrocortisone to hypotensive preterm infants due to the lack of long-term efficacy and safety data.72
Recommendation (C2): Hydrocortisone is effective in raising BP in hypotensive infants and should be used as rescue therapy for inotrope-refractory hypotension.
Inhaled nitric oxide
Inhaled nitric oxide (iNO), a selective pulmonary vasodilator, has been shown to be effective in reducing pulmonary vascular resistance and improving oxygenation in infants >34 weeks GA with persistent pulmonary hypertension of the newborn.73 Preterm infants with respiratory failure are at increased risk of pulmonary hypertension, raising the question of whether routine treatment with iNO would be beneficial in reducing mortality and other relevant outcomes. A Cochrane review found that it did not improve outcomes in preterm infants less than 34 weeks GA at risk of bronchopulmonary lung disease and was associated with a near statistically significant risk of severe cerebral hemorrhage.74 While there is no evidence of benefit from iNO in preterm infants overall, some studies suggest benefit in a subgroup of these patients. In preterm infants with a history of preterm premature rupture of membranes (PPROM), oligohydramnios, and hypoxemic respiratory failure, iNO may improve oxygenation.75
Recommendation (C2): There is no evidence to support the routine use of iNO in preterm infants at risk for bronchopulmonary dysplasia. In the rare preterm infants with documented pulmonary hypertension with right-to-left shunting and persistent hypoxemia despite optimization of ventilatory parameters, iNO may be considered, especially if there is a history of PPROM.
Conclusion
Low systemic blood flow is a common event in extremely preterm infants, particularly in the first 24 hours of life, but can be difficult to assess due to the lack of consensus markers. A comprehensive analysis of clinical, laboratory, and end-organ perfusion markers, together with echocardiographic data, should be performed to support the diagnosis and guide management. There is insufficient evidence to recommend a specific inotrope for the treatment of low systemic blood flow in preterm infants. Instead, an individualized pathophysiology-based approach is recommended.