Introduction
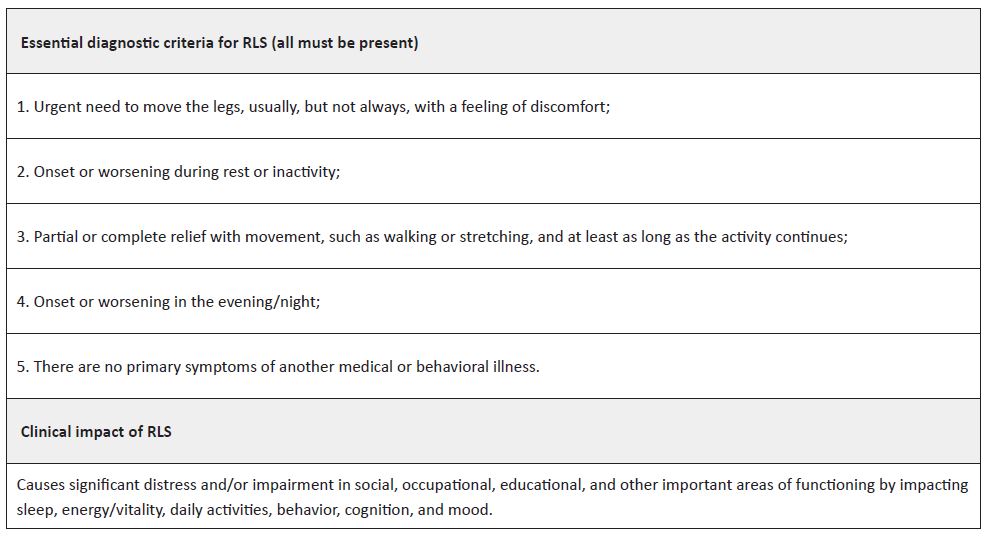
Restless legs syndrome (RLS), also known as Willis-Ekbom disease, is a neurological sensorimotor disorder that interferes with sleep, negatively affecting mood, behavior, cognition, and functional ability. In pediatric age, it has been associated with an increased incidence of several comorbidities, such as attention deficit hyperactivity disorder, parasomnias, and depressive and anxiety symptoms.1-3 Its prevalence is estimated to range between 2 and 4%, with 25−50% of cases classified as moderate to severe.1 It is characterized by the need to move the legs, often accompanied by unpleasant sensations in the lower limbs and occasionally in the arms. It usually occurs and worsens at rest and at night and is relieved by movement. The diagnosis of this clinical entity is clinical and based on the criteria established by the International Restless Legs Syndrome Study Group in 2013 (Table 1).1
Some clinical features, although not necessary to confirm the diagnosis, may be present and help support the diagnosis. These include the presence of periodic limb movements during sleep (> 5/hour) and a positive family history (RLS or periodic limb movement disorder [PLMD] in a first-degree relative or family history of periodic limb movements during sleep [> 5/hour]).
To increase the accuracy and consistency of diagnosis in children, it is essential that they report their symptoms in their own words and that the clinician is aware of the vocabulary that children and adolescents may use to describe their symptoms. They are unlikely to use terms such as “uncomfortable” or “unpleasant”. Therefore, the applicability of the criteria in Table 1 is determined by cognitive and language development, not age. In younger children, especially those under six years of age, probable RLS can be established if the symptoms are not described by the child but are observed by caregivers and meet criteria 2 to 5. The diagnosis of probable RLS may also be established if the diagnostic criteria are met but there is no worsening at the end of the day/night.1,4
Several tools have been developed to aid in the diagnosis and assessment of RLS severity, including the International Restless Legs Scale (IRLS), usually considered the gold standard, and the RLS-6 Scale of Restless Legs Syndrome/Willis-Ekbom Disease, both of which have been validated in the pediatric population.5
Genetic factors, dopaminergic dysfunction, and iron deficiency have been suggested as predominant factors in the pathophysiology of RLS.1,6 The relationship between iron deficiency and movement disorders can be explained by the role of iron as a cofactor in the rate-limiting step of dopamine production at the central nervous system level.2,8 Thus, current evidence recommends the evaluation of serum ferritin and iron supplementation when its levels are below 50 ng/dL.6
In the present study, the authors conducted a literature review to analyze the available evidence on the effect of oral iron supplementation on symptom relief in pediatric patients with RLS.
Methods
A literature search for guidelines, randomized controlled trials, systematic reviews, meta-analyses and observational studies was conducted in BMJ Evidence-Based Medicine database, Canadian Medical Association Practice Guidelines Infobase, Cochrane Library, Database of Abstracts of Reviews of Effectiveness - Centre for Reviews and Dissemination, NICE, Primary Care Clinical Practice Guidelines, and PubMed database. Articles published in Portuguese and English between 2012 and 2023 were selected. The MeSH terms “restless legs syndrome” and “iron” were used. For the PubMed search, three subcategories of the term “iron” were used to refine the search: “((“iron/administration and dosage”[Mesh] OR “iron/therapeutic use”[Mesh] OR “iron/therapy”[Mesh] )) AND “restless legs syndrome”[Mesh]”.
The criteria used to include studies in the review were defined according to the PICOS (population, intervention, comparison, outcome, and study type) model. The target population was defined as individuals of pediatric age (<18 years) with a diagnosis of RLS for whom the therapeutic intervention consisted of oral iron versus another therapeutic intervention alone versus no treatment. The outcome considered was RLS clinical improvement. Duplicate articles, classic reviews, opinion articles, and articles that were discordant with the objective of this evidence-based review were excluded. Studies that included only adults, children, or adolescents diagnosed with RLS in the context of another specific pathology, such as autism or parasomnias, and articles in which the intervention was iron administration by a route other than oral were also excluded.
The level of evidence (LE) and strength of recommendation (SR) were determined using the American Family Physician’s Strength of Recommendation Taxonomy (SORT) scale.9 This scale classifies articles according to their quality into three LEs (LE 1: studies with good-quality patient-oriented evidence; LE 2: studies with limited-quality patient-oriented evidence; LE 3: studies with non-patient-oriented but disease-oriented evidence) and three SR levels (SR A: consistent patient-oriented evidence; SR B: inconsistent patient-oriented or limited-quality evidence; SR C: consensus-based disease-oriented evidence). The inclusion and classification of articles according to the SORT taxonomy was performed independently by the authors and determined by consensus.
Results
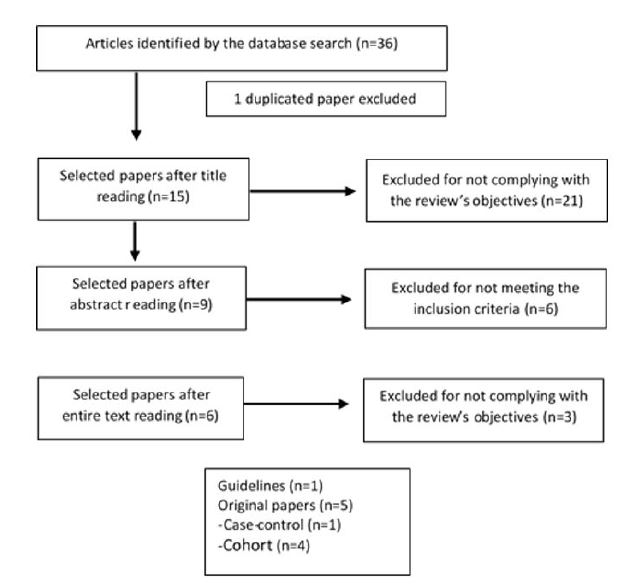
The literature search yielded 36 articles, of which six met the inclusion criteria: one guideline and five observational studies. The article selection process is detailed in Figure 1.
The guideline was developed by the International RLS Study Group in 2017 to provide guidelines for iron treatment (oral and intravenous) in adults and children with RLS and PLMD.6 Regarding the pediatric population, the guideline concluded that there was insufficient evidence to draw conclusions about the efficacy of oral or injectable iron in RLS treatment. However, it recommended that oral iron be initiated at a dose of 3 to 8.5 mg/kg/day when the ferritin level is <50ng/dL and none of the following are present: (i) conditions that are exacerbated by oral iron (e.g., inflammatory bowel disease); and (ii) conditions in which oral iron cannot be absorbed (e.g., bariatric surgery) or problems that do not allow rapid iron loss to be mitigated (e.g., heavy uterine bleeding, hereditary hemorrhagic telangiectasia, or other acquired angiodysplasia).
Treatment maintenance for three months is likely to be effective in reducing the clinical impact on sleep and RLS and PLMD symptoms. It is unclear whether treatment can be discontinued with symptom improvement or should be maintained to prevent relapse.
The five observational studies included one case-control study and four cohort studies (Table 2). The case-control study was conducted in 2013 in Japan and aimed to investigate the effect of treatment on daytime dysfunction in children with RLS.10 A group of 25 children with RLS (cases) and a group of 28 healthy children (controls) were selected, with ages ranging from seven to 18 years. Among the children with RLS, 24% had a family history of RLS in a first-degree relative. Regarding the circadian variation of symptoms, 60% of children reported both diurnal and nocturnal symptoms, and 40% reported only nocturnal symptoms. Participants were evaluated by two psychiatrists with expertise in sleep disorders and underwent polysomnography and the proposed immobilization test. Symptoms were assessed using the adult IRLS. The Japanese version of the Attention Deficit Hyperactivity Disorder-Rating Scale-IV (ADHD-RS-IV), the Pediatric Symptom Checklist (PSC), and the Pediatric Quality of Life Inventory (PedsQL™) were used to assess daytime functioning. Serum ferritin concentration was measured at baseline and a cut-off of <40 ng/mL ferritin was used to initiate supplementation. At levels >40 ng/mL, patients were treated with clonazepam or pramipexole, which were added to oral iron if there was no improvement after two months of monotherapy. At the end of the study, only eight children were receiving iron monotherapy. After three months of treatment, the study participants completed the same questionnaires and the results were compared. The authors concluded that prior to treatment, children with RLS had significantly higher scores on the ADHD-RS-IV and PSC scales and significantly lower scores on the PedsQL™. After treatment, cases showed a significant improvement in all of the aforementioned scores and similar levels of daytime dysfunction as controls. In the subgroup of children receiving iron monotherapy, 50% showed symptom resolution and the remaining 50% showed symptomatic improvement.
The retrospective cohort study conducted by Tilma and colleagues in 2012 aimed to describe the effect of oral iron treatment on RLS symptoms in early childhood.7 The study included a sample of 22 children aged 11 to 96 months who were referred for pediatric assessment for sleep disorders between 2007 and 2011. A modified version of the International Restless Legs Syndrome Study Group diagnostic criteria was used to diagnose RLS. Because eight children did not yet have the verbal skills to express the urgent need to move their legs, parental observation/opinion was considered for diagnosis. Polysomnography was performed in 14 patients, all of whom had a positive periodic limb movement index (PLMI; ≥5 movements/hour). Before oral iron treatment, serum ferritin levels were measured in 20 children, with a mean ferritin level of 21 ng/mL (range 5-42 ng/mL), but only three children had ferritin levels below the lower limit of normal for their age. Oral iron supplementation was given for one to 17 months at a dose of 5.6 mg/kg/day when ferritin was <50 ng/mL. The mean post-treatment ferritin level was 59 ng/mL. Symptom relief was dependent on ferritin concentration and significantly better in children with post-treatment ferritin >50ng/mL. A significant correlation was observed between high PLMI and low ferritin. The mean age of symptom onset was 7.5 months.
Amos et al. conducted a retrospective cohort study in 2013 to evaluate the effect of oral iron supplementation on RLS symptom relief.11 A total of 97 children, aged five to 18 years, able to verbalize symptoms, and with a diagnosis of RLS (ICD-9 diagnosis code 333.94) were included. Data were reviewed by two sleep specialists. The mean baseline ferritin level was 22.7 ng/mL, with 71% of children having a ferritin level <30 ng/mL. Most children (63 of 97) received oral iron supplementation at a dose of 3.5 mg/kg/day (when ferritin was <50 ng/mL) as monotherapy or in combination with other treatments. The remainder received nonpharmacologic interventions or other pharmacologic treatments: melatonin, gabapentin, clonidine, or dopamine agonists (pramipexole or ropinirole). A total of 75 children were followed up. Of these, approximately 80% (n=59) of those who received oral iron showed symptom improvement or resolution (with a mean time to symptom improvement of 3.8 months) compared to only 43.8% of children who did not receive oral iron.
In 2019, Rosen et al. conducted a prospective cohort study to determine whether oral iron supplementation induced RLS symptom improvement in a pediatric population.12 A total of 117 patients aged five to 18 years with RLS symptoms and ferritin <50 ng/mL were initially enrolled, but only 47 were included in the final sample. RLS diagnosis was established using the International Restless Legs Syndrome Study Group criteria. After diagnosis, patients received ferrous sulfate (3 mg/kg/d) plus vitamin C (250 mg) supplementation for eight weeks. At follow-up, a significant increase in ferritin levels (from 23 ng/mL at baseline to 40 ng/mL) and a relevant change in RLS score were observed. However, no significant correlations were observed between baseline RLS scores and ferritin levels or in the final values. The study authors concluded that iron deficiency/low serum ferritin levels are common in children with restless sleep and RLS and that, although there was no statistically significant correlation, correction of iron deficiency improved symptoms in children with this syndrome, and therefore correction of low serum ferritin levels should be recommended in these patients.
The last observational study was a retrospective cohort study to evaluate the long-term effect of oral iron supplementation (3 mg/kg/day) on ferritin levels and RLS symptom improvement.13 The study was based on the records of the Pediatric Clinic where children with RLS were already being followed and iron supplementation was the first-line therapy when ferritin levels were <50 ng/mL. A total of 105 children were included, 41 with RLS, with ferritin levels <50 ng/mL and receiving oral iron supplementation (3 mg/kg/day). Four follow-up periods were defined - three months, three to six months, one to two years, and > two years post-iron supplementation -, in which serum ferritin and iron levels and symptom changes were evaluated. Results showed that 63% of children had sustained symptom improvement after oral iron supplementation, but 78% had subjective symptom improvement. Ferritin levels increased in 66%, 90%, and 100% of children at each of the considered follow-up periods, respectively. The authors concluded that oral iron supplementation promoted a sustained improvement in serum ferritin levels and RLS symptoms in children and adolescents.
Table 2 Brief description of the observational studies included in the review.
| Authors | Type of study | Sample size | Treatment duration | Initial ferritin level (mean) | Iron supplementation dose and formulation | Outcomes | Conclusions | LE |
| Naomichi Furudate et al. | Case-control | Experimental group: 25 children Control group: 28 children (7-18 years) | 3 months | 29.7±19.1 ng/mL | 50 mg/day (in association with other drugs) or 56.25 mg/day (in monotherapy); sodium ferrous citrate | Evaluate daytime dysfunction in children with RLS and the effects of iron treatment on RLS symptoms and daytime dysfunction. | Children with RLS before treatment had significantly higher total scores on the ADHD-RS-IV, total PSC- and ADHD scales. There was a significant improvement in scores in the RLS group after treatment. After treatment, participants' daytime function showed similar levels to controls. | LE2 |
| Jens Tilma et al. | Retrospective cohort | 22 children (11-96 months) | 1-17 months | 21 ng/mL | 3.6-8.5 mg/kg/day | Symptoms relief was recorded and correlated with ferritin levels and PLMS index levels. | After oral iron treatment, an increase in ferritin levels was observed in all cases. Symptom relief was dependent on ferritin concentration and significantly better in children with ferritin levels after treatment >50 ng/mL. | LE2 |
| Louella B. Amos et al. | Retrospective cohort | 97 children (5-18 years) | Median duration: 3.8 months | 22.7 ng/mL | 3-4 mg/kg/day; ferrous sulfate | Symptoms relief or resolution. | 80% of children treated with oral iron had symptom improvement or resolution vs. 43.8% of children not treated with oral iron. Children treated with oral iron were significantly more likely to experience symptom relief or resolution vs. children not treated with oral iron (p=0.01). | LE2 |
| Gerald M. Rosen, MD et al. | Prospective cohort | 47 children (5-18 years) | 2 months | 23 ng/mL | 3 mg/kg/day; ferrous sulfate | Determine if oral iron supplementation improved restless legs/restless sleep symptoms in a pediatric population. | A non-statistically significant improvement was observed in RLS symptoms, but also a significant increase in ferritin levels. | LE2 |
| Thomas J. Dye et al. | Retrospective cohort | 105 children (41 with RLS and 64 with PLMD) Mean age: 10.2±5.3 years | According to ferritin levels, which were measured every 3-6 months | 27.4 ±12.1 ng/mL | 3 mg/kg/day; ferrous sulfate | Evaluate the long-term effect of iron supplementation on ferritin levels and RLS symptom improvement. | In long-term follow-up (>2 years after iron therapy), a sustained improvement was observed in ferritin levels and RLS symptoms in the RLS cohort. Iron therapy appeared to lead to a long-lasting improvement in children with RLS. | LE2 |
ADHD - Attention deficit hyperactivity disorder; ADHD-RS-IV - Attention deficit hyperactivity disorder rating scale IV; LE - Level of evidence; PLMD - Periodic limb movement disorder; PLMS - Periodic limb movements of sleep PSC - Pediatric symptom checklist; RLS - Restless leg syndrome
Discussion
While the exact cause of RLS in children remains unclear, evidence suggests a possible association with iron deficiency. This review highlights the potential benefits of oral iron supplementation in children with RLS. Several studies have reported improvements in RLS symptoms with iron therapy, suggesting a possible role for iron in the pathogenesis of RLS. However, more research is needed to determine the optimal dosing regimen and treatment duration, as well as the long-term effects of iron supplementation. Clinicians should consider iron deficiency as a potential underlying cause of RLS and individualize treatment based on a comprehensive patient assessment and monitoring.
When interpreting the findings of studies examining the potential benefits of oral iron supplementation in children with RLS, it is important to consider potential biases that may affect the validity and reliability of the results. One potential bias arises from the reliance on parental self-report of children’s symptom improvement. This subjective measure introduces the possibility of recall bias, as parents may overestimate or underestimate the degree of improvement based on their own perceptions and expectations. On the other hand, the lack of a control group in some studies limits the ability to compare the effects of iron supplementation with a placebo or alternative treatment, making it difficult to determine the specific contribution of iron. Another bias arises from the small sample size in some studies. Small sample sizes reduce statistical power and can lead to imprecise estimates of treatment effects. This can affect the generalizability of study results to a larger population. On the other hand, small sample sizes may increase the risk of random variation and make it difficult to detect meaningful differences or determine the true effectiveness of iron supplementation. Therefore, caution should be exercised when interpreting the results of studies with small sample sizes. Future research should aim to incorporate objective measures, randomized controlled designs, and independent assessment of symptoms to mitigate these potential biases and provide more robust evidence of the benefits of oral iron supplementation in children with RLS.
Conclusion
Given the limited number of studies on the topic and their limitations, a B-level SORT strength of recommendation was assigned to the use of oral iron supplementation as treatment of RLS when ferritin levels are below 50 μg/dL. Overall, the available evidence suggests that iron supplementation at doses of 3-8.5 mg/kg/day for a period of 12 weeks may be beneficial in controlling pediatric RLS symptoms. However, further studies with larger sample sizes and longer follow-up are needed to determine more accurately what the actual ferritin level should be, as well as other markers of iron kinetics that may be useful in predicting symptom improvement in pediatric patients. Randomized controlled trials are also needed to better determine the dose and duration of treatment, as well as the best route of iron administration and indications for initiating intravenous therapy.