Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portugaliae Electrochimica Acta
Print version ISSN 0872-1904
Port. Electrochim. Acta vol.28 no.2 Coimbra 2010
Study of the Anticorrosive Properties of Different Bi-Layers of Polypyrrole - BTSE on Carbon Steel Immersed in NaCl Media
A.L. Correa-Borroel,1,2 S. Gutierrez,2 E. Arce Estrada,3 R. Cabrera-Sierra,4 P. Herrasti1,*
1 Universidad Autónoma de Madrid. Departamento de Química-Física Aplicada 28049 Madrid, Spain
2 Departamento de Química, Universidad de Guanajuato, Cerro de la Venada s/n 36040 Gto, México
3 Instituto Politécnico Nacional, Escuela Superior de Ingeniería Química e Industrias Extractivas, Departamento de Metalurgia. UPALM Ed. 7, C.P. 07738, México, D.F.
4 Instituto Politécnico Nacional, Escuela Superior de Ingeniería Química e Industrias Extractivas, Departamento de Ingeniería Química Industrial. UPALM Ed. 7, C.P. 07738, México, D.F.
DOI: 10.4152/pea.201002113
Abstract
To improve the corrosion resistance of mild steel, in this work, bi-layers of BTSE (bis-(triethoxysilyl) ethane) and Ppy coatings have been deposited via adsorption and electrodeposition, respectively. The anticorrosive properties of these bi-layers have been evaluated in an aggressive medium like 3% NaCl, and compared with the layers of BTSE and Ppy in a separate way. In spite of the aggressive solution, it was evaluated a poor behaviour of both Ppy and BTSE coatings using potential measurements, EIS and SEM techniques. A better behaviour was expected because the preparation of the bi-layers Ppy/BTSE and BTSE/Ppy showed a protective behaviour using SEM and salt spray tests. However, EIS results predicted an evolution of these coatings for long immersion times being evident the oxidation of the steel.
Keywords: bi-layers, corrosion, impedance, organosilane, polypyrrole.
Introduction
Steel is a common industrial material and its study against corrosion is a topic of research in continuous development. OneOne of the most common methodologies to prevent corrosion is the application of organic compounds, including paints. These paints protect the metal for long periods of time, in many cases containing inhibitors in their composition, which makes an increase in durability. The biggest drawback is the permeability, which causes delamination of the paints and lose all their corrosion resistance. To avoid this kind of damage, a type of compounds started to be used; among them, organosilanes, which allow the anchorage between the organic compound used as a protector, and the substrate. Recent publications showed that the conversion coatings formed by silane coupling agents are comparable to or better than the chrome conversion coatings and can provide excellent seats for paint adhesion Recent publications have showed that the conversion coatings formed by silane coupling agents are comparable to (or better than) the chrome conversion coatings, and can provide excellent seats for paint adhesion [1-3].
In general, silanes such as the bis-(triethoxysilyl) ethane (BTSE) have a very limited stability in water because of hydrolysis and condensation. Therefore, a high amount of organic solvents is needed in the preparation of solutions of multifunctional silanes. The stability, hydrolysis, and condensation kinetics of a typical bis-silane BTSE in water-ethanol-silane solution have been investigated by Pu et al. [4] with excellent results at pH 4.5. Some authors report that the BTSE film is more rigid and has lower diffusion rates for water and ions than other organosilanes [5].
Alternative compounds of the paintings that have an additional property for control and decrease the corrosion rate, are the conducting polymers. These materials have been used in many fields, due to their extraordinary properties. One of them is the ability to act as a regenerative passive film due to its redox properties. The polypyrrole (Ppy) is one of the most commonly used conducting polymers, due to its easy preparation, stability and other properties. Thus, it is very important to select of the anions which promote passivation of the metal surface in contact with the electrolyte. The electrodeposition of Ppy or any other conducting polymer is relatively simple on inert metals (Pt, Au, GC, etc.); on non-inert metals, such as Cu, Ti, Fe, etc. [6], it is needed for electrodeposition that the substrate does not dissolve simultaneously during the electropolymerization process. Thus, it is very important to select the anions which promote passivation of the metal surface in contact with the electrolyte.
IIn this paper we have addressed the preparation of polypyrrole layers on carbon steel using two types of electrolyte: nitric acid, with a high oxidizing power, and oxalic acid, that produces a protective passive film of iron oxalate [7]. In the same way, we have studied the organosilane adsorption of BTSE. The anticorrosive properties of both layers are evaluated immersed in 3% NaCl using electrochemical techniques. To increase the stability of the coatings, bi-layers of Ppy/BTSE and BTSE/Ppy on carbon steel have been obtained, and their morphological characterization and anticorrosive properties have been studied.
Experimental
The working electrode was a carbon steel specimen with composition: 97.4% Fe, 1% C, 0.90% Mn, 0.5% Si, 0.10% P and 0.10% S, which was polished with emery paper, washed with distilled water and degreased with acetone before used. Its electrode area was approximately 2 cm2. The electrochemical measurements were performed using a PAR VerSat potentiostat or an AutoPG UAM potentiostat. The carbon steel electrodes previously modified were immersed in a 3% NaCl.
Preparation of organosilane layer (steel / BTSE)
The organosilane used was the BTSE, Bis 1,2 (triethoxysilyl) ethane, pure liquid Aldrich products. Silane should be hydrolysed before its application in order to form enough Si-OH groups in the presence of water and methanol, using 86 mL of methanol, 6.6 mL of water, 6.6 mL of BTSE and 0.66 mL of acetic acid (pH ≈ 4.5), and stirred for 24 hours [8]. Organosilane hydrolysis depends on the concentration, pH of the medium, temperature and aging itself [9]. The temperature was held constant at 25° C and the solution was prepared each time it was used. For the adsorption of the organosilane the substrates were immersed for 1h and thermally treated for 30 min at 80 ºC. This treatment was necessary to achieve the condensation of the hydrolyzed organosilane on the surface and its adhesion with the formation of M-O-Si bonds.
Preparation of polypyrrole layer (steel / Ppy)
The counter electrode for the electrodeposition of pyrrole was the proper cell of stainless steel and an Ag/AgCl (3M) was used as reference electrode. Pyrrole (Aldrich) was distilled and then stored in a refrigerator. Nitric and oxalic acids were used as supporting electrolyte in concentrations 0.1 and 0.3 M, respectively. The Ppy was electrodeposited using cyclic voltammetry technique by applying a potential scan from 0 to 1.4 V at a scan rate of 100 and 10 mVs-1 in nitric and oxalic acids, respectively. The pyrrole concentration was maintained in all cases to 0.5 M.
Preparation of bi-layersAcero/silano/Ppy steel/silane/Ppy and steel/Ppy/silane
The preparation of these bi-layers is carried out following the procedure described below. For steel/silane/Ppy, the organosilane layers are previously formed by immersion in the organosilane solution and this substrate was used as working electrode for the electrodeposition of polypyrrole in 0.1 and 0.3 M of nitric and oxalic acids, respectively, using three scans. In the case of bi-layers polypyrrole was electrodeposited on the mild steel substrate using cyclic voltammetry and the organosilane was adsorbed on the top of it.
Morphological characterization of deposits
The morphology of the deposits was examined with a scanning electron microscope Phillips XL30EDAX PV 9900.
Anticorrosive studies
Linear polarization curves and Electrochemical Impedance Spectroscopy techniques were used for studying the anticorrosive properties of the steel modified by different bi-layers immersed in NaCl solution. Polarization curves were recorded separately in the positive and negative direction by scanning the potential from open circuit potential at 2 mVs-1. The EIS measurements were obtained using a sinusoidal signal of 10 mV amplitude with respect to the open circuit potential from 10000 Hz to 0.01 Hz. Besides accelerated corrosion studies were conducted using a salt spray chamber.
Results and discussion
Ppy films electrodeposition
Fig. 1a and 2a show the typical voltammograms obtained during the formation of polypyrrole (Ppy) films on carbon steel in nitric and oxalic acid solutions, respectively. Fig. 1a shows higher current compared with Fig. 2a during the electropolymerization process. The mechanism of polypyrrol growth in nitric acid medium occurs through the oxidation of the surface. The oxidation produces small pits that serve as nucleation points [10,11], both processes generating an increase in current higher than in the case of oxalic acid as electrolyte. The voltammogram in Fig. 2a is quite different; it shows a broad oxidation peak due to the oxidation of the carbon steel to Fe2+ and the polymerization of pyrrol. The Fe2+ forms a passive layer with the oxalate in solution given iron oxalate [13]. In the reverse scan a second oxidation peak appears; it is due to the oxidation of the metal that has not been covered by polypyrrol. Successive scans show a decrease of these peaks due to the polypyrrole coating. The lower current in this medium indicates that once the passive layer is formed, the current in the voltammogram only corresponds to the oxidation of pyrrol in solution. The thickness of polypyrrol electrodeposited in both medium is very similar (~ 2µm). The number of cycles to obtain this thickness in nitric acid solutions is higher than in oxalic acid solution. This difference is explained by the different electropolymerization mechanism. In the SEM images it is evident the porous nature and the globular morphology of the Ppy formed on the carbon steel in nitric acid solution (Fig. 1b); meanwhile, in oxalic acid (Fig. 2b) the globular size of the polymer is smaller and has some aglomerations.
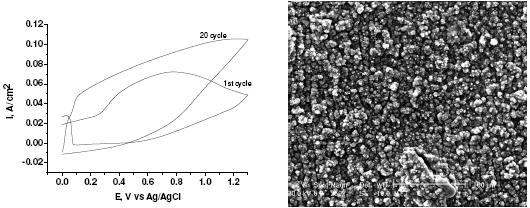
Figure 1. a) Cyclic voltammograms obtained during the deposition of Ppy films on carbon steel electrode in 0.1 M nitric acid and 0.5 M pyrrol, scan rate: 100 mVs-1; b) SEM image recorded after the formation of Ppy.
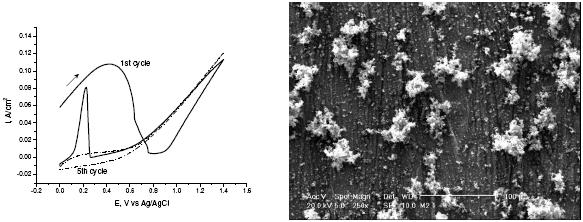
Figure 2. a) Cyclic voltammograms obtained during the deposition of Ppy films on carbon steel electrode in 0.3 M oxalic acid and 0.5 M pyrrol, scan rate: 10 mVs-1; b) SEM image recorded after the formation of Ppy.
The open circuit potential against time for Ppy films formed in both electrolytes (nitric and oxalic acid) and the carbon steel immersed in the NaCl solution are shown in Fig. 3. The Ecorr show an exponential decayment within the first hour; it is due to conformational rearrangements induced by Cl- ion penetration and polymer reduction, the latter associated with the regeneration of the Fe oxide layer at the coating/metal interface. The observation suggests that the Ppy film does not behave as a simple barrier against aggressive species, but reflects anodic protection. Ppy corrosion is typically associated to the so-called “ennobling” mechanism [14-16], where the polymer provides anodic galvanic protection by keeping the metal passive at small defects or acting as an oxidized, thus improving the oxide layer at the metal/Ppy interface.
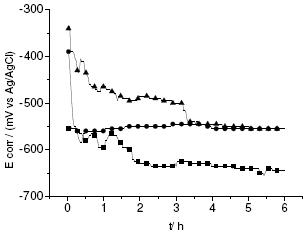
Figure 3. Variation of the open circuit potential for the Ppy films formed in nitric and oxalic acids vs. time. Steel ■, Steel/Ppy ox ▲ and Steel/Ppy HNO3 ●
In both cases the behaviour is very similar; the anodic activity produces a rapid current decrease, and then stabilization of the current before breakdown of the film occurs. In the case of the film prepared using nitric acid the breakdown occurs later.
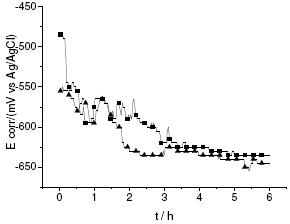
Figure 4. Variation of the open circuit potential for the carbon steel and a BTSE film on carbon steel vs. time.
BTSE films formed on carbon steel
A visual inspection of the BTSE fims formed by immersion on carbon steel appeared not uniform, indicating poor wetting of the metal surface by MeSi hydrolyzed solution. To evaluate the stability of the different coatings, the variations of the open circuit potential of the modified steel inmersed in NaCl solutions has been done. It is observed a shift in the potential towards more negative values, varying from -550 to -650 mV, being very similar to those evaluated for the bare steel immersed in the same solution (see Fig. 4). This fact could indicate that the coatings of BTSE are poor against the oxidation of the steel exposed to an aggressive solution. This is supported by the linear polarization curves (not shown), where it is observed a similar electrochemical response and slightly variations in the current magnitudes, in comparison with those evaluated for the carbon steel, which is attributed to poorer adhesion of the coating on the surface that leads to inefficient bonding of silane.
Bilayers BTSE/Ppy and Ppy/BTSE formed on carbon steel
Fig. 5 shows an image of the steel/BTSE/Ppy, where it is evident the presence of an internal BTSE film, aproximatly 10 µm in thickness, and the globular morphology of Ppy.
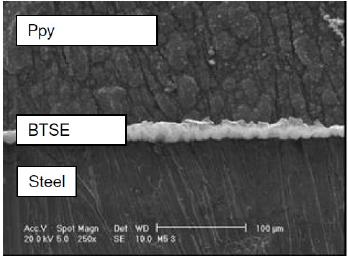
Figure 5. A SEM image obtained for the bi-layer BTSE/Ppy formed on carbon steel in 0.1 M nitric acid and 0.5 M pyrrol solution.
In Fig. 6, it is displayed the variation of the open circuit potential for the different bi-layers formed on the carbon steel surface trough the immersion time in NaCl medium. In this plot the potentials recorded for the bi-layers are very similar to those reported for the bare steel in the same solution. The bi-layers that have Ppy on the top seem to have best behaviour against corrosion. These variations could be related with the anticorrosive properties of the coatings. The linear polarization curves (no shown) obtained for the different bi-layers showed a similar behavior as well as a noticeable decrease of 4 times of magnitude in the current values.
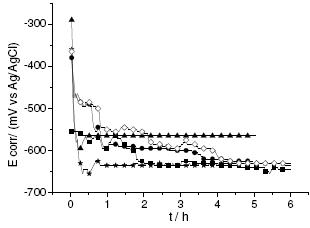
Figure 6. Variation of the open circuit potential for the bi-layers of Ppy and BTSE films vs. time. Steel ■, Steel/BTSE/PPyHNO3 ●, Steel/BTSE/PPyOX ▲, Steel/PPyHNO3 / BTSE * , Steel/PPyOX/BTSE ◊.
As a first attempt to support the anticorrosive properties of the Ppy, BTSE and bi-layers, they were introduced in a salt fog cabinet during 7 days. After the exposure of the coupons, they were characterized by SEM and the micrographs are shown in Fig. 7. The most important damage of the steel is observed on the bare steel, being evident a non uniform damage (Fig. 7a) as a consequence of the aggressiveness of the solution. However, in presence of the BTSE and Ppy/BTSE, the oxidation of the steel decreases, being evident the formation of a roughness and porous steel surface (Fig. 7b); meanwhile, in Fig. 7c and 7d the SEM images for the bi-layers BTSE/Ppy and Ppy/BTSE, suggested a better barrier behavior of the coating in the oxidation of the steel. This fact could be associated with the passage of aggressive ions through the coatings, i.e., oxygen, H2O, and Cl-.

Figure 7. SEM images obtained after the exposition of the steel in the salt fog cabinet. a) bare steel, b) steel/PpyOX, c) steel/PpyOX/BTSE and d) steel/BTSE/PpyOX.
Table 1 shows the porosity values of the coatings before and after they are introduced in the spray salt chamber [17] . The porosity is lower when the bi-layers are deposited on the carbon steel than polypyrrole coating. The smaller value is obtained for the bi-layer BTSE/Ppy (1.18x10-7) and this value increases in two orders of magnitude after the corrosive treatment (8.69x10-5). This behaviour confirms the best anticorrosive properties of the bi-layers with the Ppy on top.
Table 1. Porosity values of different substrates before and after a salt fog chamber.

EIS characterization of Ppy and BTSE films formed on carbon steel
To discuss the oxidation process of mild steel with and without coatings a simulation of the impedance diagrams obtained experimentally has been done using the equivalent circuit shown in Fig. 8. This circuit considers three time constants: Rs = solution resistance, Qc and Rc = capacitance and resistance due to the coating, Qdl and Rct = capacitance of the double layer and resistance to the charger transfer, Qd and Rd = impedance response to the diffusion of oxygen.
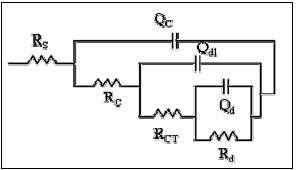
Figure 8. Equivalent circuit used for fitting the EIS diagrams shown in figures 9 and 10.
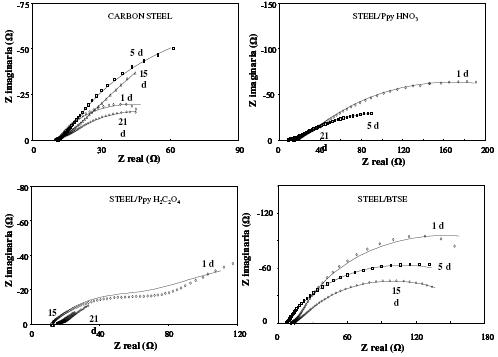
Figure 9. Typical EIS diagrams obtained at different immersion times of the steel in 3% NaCl. a) bare steel, b) steel/PpyHNO3, c) Steel/PpyH2C2O4 and d) steel/BTSE.
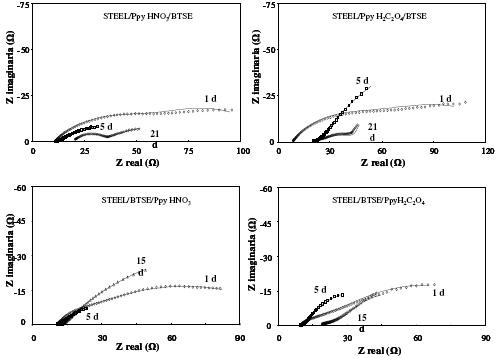
Figure 10. Typical EIS diagrams obtained at different immersion times of the steel in 3% NaCl. a) Steel/Ppy HNO3/BTSE, b) steel/PpyOX/BTSE, c) steel/BTSE/PpyHNO3 and d) steel/BTSE/PpyOX.
In Fig. 9, it is shown the Nyquist diagrams for the carbon steel in presence of Ppy formed in nitric and oxalic acids, BTSE as well as the bare steel through the immersion in 3% NaCl solution. In these diagrams it is evident the modification of the complex plots through the time. Most of them exhibit the maxima values for shorter immersion times, suggesting an evolution in the coverage extent of the coatings. It is important to note that the smallest impedance values are observed for the bare steel and in presence of Ppy formed in nitric and oxalic acids, indicating an increase in the active oxidation of the steel. This means that the protection properties of these layers are very poor, fact already evidenced by the SEM images shown in Fig. 7. In spite of this active behavior of the steel, it can be seen in the Nyquist plots that in the presence of BTSE, impedance values at least in one order of magnitude are observed. Based on these results, it seems that the damage of the coating and the oxidation of the steel are very active for longer immersion times. This fact is due to the aggressiveness of the solution, as well as to the immersion time, in comparison with the results evaluated using the fog cabinet for 7 days.
EIS characterization of bi-layers Ppy - BTSE films formed on carbon steel
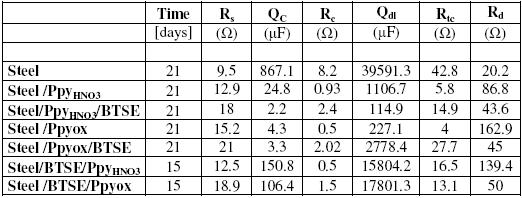
Fig. 10 presents the EIS diagrams obtained in presence of bi-layers Ppy/BTSE and BTSE/Ppy through the immersion in the corrosive solution. In these diagrams a similar behavior to those evaluated for the same coatings in a separate way is evident. Firstly it is observed that the spectra recorded at 1d show the maxima impedance values; meanwhile, for longer times they decrease. As already mentioned, this behavior is related to modifications in the barrier properties of the bi-layers. It is interesting to note that the active condition is very similar for the different coatings, still in the case of BTSE/Ppy. It seems that the protective behavior of both Ppy and BTSE is dependent on the immersion time. These results mean that the assembly BTSE/Ppy and Ppy/BTSE is very poor against the corrosion of the steel. Probably these problems could be related to the oxidation of the steel during the Ppy formation, because it has been reported a beneficial behavior when they are formed on aluminum [18]. Table 2 shows the parameters obtained from the EIS diagrams. The values of Rs for all coatings are very similar (same order of magnitude); only the carbon steel shows a lower value (9.5 Ω). The values of Qc are not very high; this is probably due to modifications in the coating with the immersion time. The low values corresponding to the coating resistance suggest their detachment. The double layer capacitance increases in the carbon steel, due to the generation of a passive layer that decreases the active area. The resistances of the charge transfer have values corresponding to materials with high porosity. The porosity of these coatings allow the ions to reach the surface and promote the oxidation of the mild steel. The resistance to the oxygen diffusion is very low, suggesting the formation of an oxide layer for all cases.
Table 2. Values of the parameters involved in the simulation of Figs. 9 and 10. The parameters have been calculated using the Boukamp program.
Conclusions
The study of the corrosion of BTSE and polypyrrole coatings on mild steel has demonstrated very limited protection. The formation of bi-layers BTSE/Ppy and Ppy/BTSE produces a decrease in the corrosion rate. The OCP potential is more stable in BTSE/Ppy layers. The value is stabilized at -550 mV, 100 mV more positive than the OCP in mild steel. These bi-layers have been used in a corrosive medium as a salt spray chamber, showing a good behavior against corrosion pitting. In spite of the bi-layer BTSE/Ppy has the best properties, adherence and low porosity (8.69 x 10-5), it could be used as a prospective coating of mild steel; however, using EIS technique showed a fair protection of these coatings when they are exposed to a very aggressive medium as 3% of NaCl for a long period of time.
References
1.T.F. Child, W.J. Van Ooij, Trans. Inst. Met. Finish. 77 (1999) 64-69.
2.N. Tang, W.J. Van Ooij, G. Górecki, Prog. Org. Coat. 30 (1997) 255-263. [10.1016/S0300-9440(96)00691-1]
3.W.J. Van Ooij, T. Child, Chemtech. Vol. (1998) 26.1.- H.
4.Z. Pu, W.J. Van Ooij, J.E. Mark, J. Adh. Sci. Technol. 11 (1997) 29-47.
5.D. Zhu, Ph.D. Dissertation, Department of Material Science and Engineering, University of Cincinnati, 2002.
6.M. Schirmeisen, F. Beck, J. Appl. Electrochem. 19 (1989) 401-409. [10.1007/BF01015243]
7.S. Wencheng, J.O. Iroh, Electrochim. Acta 46 (2000) 1-8. [10.1016/S0013-4686(00)00518-1]
8.T. Van Schaftinhen, C. Le Pena, H. Terryn, F. Hörzenberger, Electrochim. Acta 49 (2004) 2997-3004. [10.1016/j.electacta.2004.01.059]
9.E.P. Plueddemann, Silane Coupling Agents, 2nd edn. Plenum, New York, 1991.
10.G. Mengoli, M.M. Musiani, Electrochim. Acta 31 (1986) 201-210. [10.1016/0013-4686(86)87109-2]
11.F. Beck, R. Michaelis, F. Schloten, B. Zinger, Electrochim. Acta 39 (1994) 229-234. [10.1016/0013-4686(94)80058-8]
12.G.S. Akundy, J.O. Iroh, Polymer 42 (2001) 9665-9669. [10.1016/S0032-3861(01)00529-8]
13.M.A. Arenas, L. Gónzalez Bajos, J.J. de Damborenea, P. Ocón, Prog. Org. Coat. 62 (2008) 79-86. [10.1016/j.porgcoat.2007.09.019]
14.J. Reut, A. Öpik, K. Idla, Synth. Met. 102 (1999) 1392-1393. [10.1016/S0379-6779(98)01036-4]
15.T.D. Nguyen, M. Keddam, H. Takenouti, Electrochem. Solid State Lett. 6 (2003) B25-B28. [10.1149/1.1582252]
16.B. Wessling, Mat. Corros. 47 (1996) 439. [10.1002/maco.19960470804] [ Links ]
18.A.L. Correa-Borroel, S. Gutierrez, E. Arce, R. Cabrera-Sierra, P. Herrasti, J. Appl. Electrochem. 39 (2009) 2385-2395. [10.1007/s10800-009-9925-z]
Received 15 December 2009; accepted 17 May 2010
* Corresponding author. E-mail address: pilar.herrasti@uam.es














