Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.30 no.6 Coimbra nov. 2012
https://doi.org/10.4152/pea.201206427
Electrobioremediation of Patagonian Soils Contaminated with Hydrocarbons
H.E. Megahed*
Department of Chemistry, Faculty of Science; Benha University, Benha, Egypt.
Abstract
The effect of 2-amino-4,6-dihydroxy pyrimidine (ADHP) on the corrosion of carbon steel in 1 M HCl has been studied using weight loss and galvanostatic polarization measurements. The percentage inhibition efficiency was found to increase with increasing concentration of the inhibitor, and with decreasing temperature. The inhibitive effect of these compounds was explained on the basis of the formation of an insoluble complex adsorbed on the metal surface. The adsorption process follows Langmuir adsorption isotherm. The effect of temperature on the rate of the corrosion in the absence and presence of these compounds was also, studied. The activated thermodynamic parameters were calculated and explained.
Keywords: corrosion test, C-steel, polarization.
Introduction
Acid solutions are generally used for the removal of undesirable scale and rust in several industrial processes. Hydrochloric acid is widely used in the pickling process of steel. The use of inhibitors is one of the most practical methods for protection against corrosion especially in acid solutions to prevent metal dissolution and acid consumption [1]. Most well known acid inhibitors for steel corrosion are organic compounds containing [2-10]. It has been observed that most of the organic inhibitors act by adsorption on the metal surface [11].
The adsorption of corrosion inhibitor depends mainly on physical chemical properties of the molecules such as functional group, steric factor, molecule size, molecular weight, molecular structure, aromaticity, electron density at the donor atoms and π-orbital character of donating electrons [12].
The present investigations aim to study the effect of 2-amino-4,6-dihydroxy pyrimidine (ADHP) as corrosion inhibitors on the corrosion of 1018 carbon steel in 1 M HCl using weight loss and galvanostatic polarization measurements. The effect of temperature on the dissolution of carbon steel in 1 M HCl containing the inhibitors used was also studied and some thermodynamic parameters were computed.
Experimental method
Carbon steel of type 1018 used in this study has the chemical composition C 0.2%, Mn 0.6%, P 0.04 %, Si 0.003% and the remainder iron. Weight loss measurements were performed using compounds of the dimensions (3 × 3 × 0.1) cm3. For galvanostatic polarization, a cylindrical rod embedded in araldite with exposed surface area of 0.2 cm2 was used. The electrodes were polished with different emery papers, degreased with acetone and rinsed by distilled water, before being inserted in the test solution. For weight loss measurements, the steel coupons were left hanged in the test solution (1 M HCl) for 150 minutes at 25 ± 1 °C before recording the loss of their weight.
The galvanostatic cathodic and anodic polarization measurements were carried out using a three-compartment glass cell and an EG&G model 363 potentiostate/galvanostate corrosion measurement system. Platinum electrode was used as a counter electrode (separated from the cell solution by a sintered glass frit) and a saturated calomel electrode (inside a luggin's probe) as a reference electrode.
The inhibition efficiency IE was calculated using the following equation:
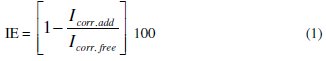
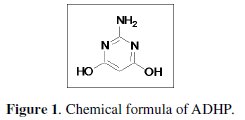
where Icorr.add and Icorr.free are the corrosion rates in free and inhibited acid, respectively. The general formula of 2-amino-4,6-dihydroxy pyrimidine(ADHP) is represented in Fig. 1.
Results and discussion
Weight loss measurements
The corrosion of carbon steel in 1 M HCl in the absence and presence of different concentrations of ADHP was studied by weight loss experiments. The corrosion rate (Rcorr) and the percentage protection efficiency IE (%) were calculated using equations 2 and 3, respectively [13, 14]:

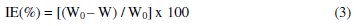
where Δm (mg) is the weight loss, S (cm2) is the surface area, t (h) is the immersion time, and W0 and W (mg.cm-2.h-1) are the corrosion rates of carbon steel in the absence and the presence of the inhibitor, respectively. The values of inhibition efficiencies and corrosion rates obtained from weight loss measurements with the addition of different concentrations of ADHP after 24 h immersion in 1 M HCl solutions at various temperatures are listed in Table 1.
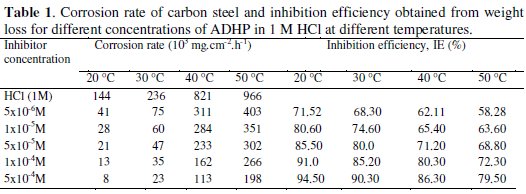
Inspection of Table 1 reveals that as the inhibitor concentration is increased, the corrosion rate decreases while the inhibition efficiency increases. This behavior could be attributed to the increase of the number of adsorbed molecules at the metal surface.
Adsorption isotherm
Since the corrosion inhibition process is based on the adsorption of the inhibitor molecules on the metal surface, the degree of surface coverage θ for different concentrations of ADHP at different temperatures was evaluated by the weight loss method by using the following equation:

To study the adsorption behavior of ADHP on 1018 carbon steel in 1 M HCl, the adsorption isotherm must be defined. Attempts were made to fit θ values to various isotherms including Frumkin, Langmuir, Temkin and Freundlich isotherms. By far the results were best fitted by Langmuir adsorption isotherm.
Langmuir adsorption isotherm could be represented using the following equation:

where K and C are the equilibrium constants of adsorption process and additive concentration, respectively.
The linear regressions between C/θ and C for each temperature were drawn (Fig. 2) and the parameters are listed in Table 2.
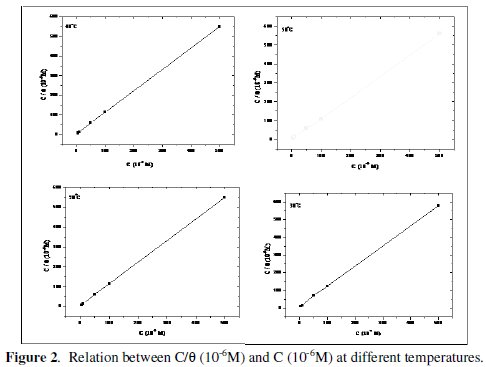
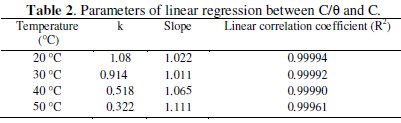
The results show that all the linear correlation coefficients and all the slopes are close to unit, which confirms that the adsorption of ADHP in 1 M HCl follows Langmuir adsorption isotherm. The considerable deviation of the slopes from unity shows that the isotherm cannot be strictly applied. This deviation is attributable to the interaction between adsorbed species on the metal surface [15].
The values of (K) decrease with an increase in temperature (Table 2), indicating that the interaction between the adsorbed molecules and the metal surface is weakened and consequently, the adsorbed molecules become easily removable. Such data explain the decrease in the protection efficiency with increasing temperature.
Thermodynamic parameters
The fundamental thermodynamic functions are very important to explain the adsorption phenomenon of the inhibitor molecules. Using the obtained adsorption coefficients, the heat of adsorption, free energy of adsorption and adsorption entropy can be calculated.
The heat of adsorption was calculated according to the Langmuir adsorption isotherm [16] which can be expressed by:
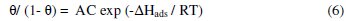
where, T is temperature, A is an independent constant, C is the inhibitor concentration, R is the gas constant, ΔHads is heat of adsorption and θ is the surface coverage by the inhibitor molecule. Eq.(6) can be converted to logarithmic scale
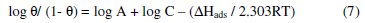
The values of heat of adsorption can be calculated from the slope of the relationship between log( θ/(1-θ) ) and (1/T) (Fig. 3).
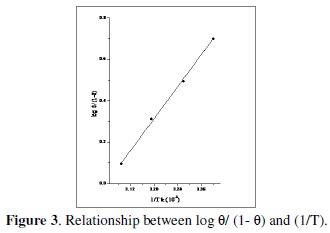
Straight line relationships were obtained. This means that the adsorption of ADHP on the steel surface obeys the Langmuir adsorption isotherm. The standard free energy of adsorption, (ΔG0ads), was calculated from the equilibrium constant of adsorption using the following equation
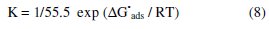
Then the standard adsorption entropy (ΔS0ads) can be obtained using the thermodynamic fundamental equation [17]:

All the obtained thermodynamic parameters are shown in Table 3.
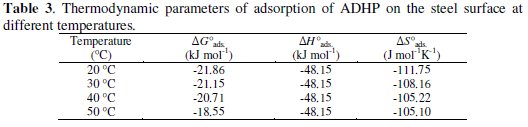
The negative values of ΔG0ads suggested that the adsorption of ADHP onto the steel surface was a spontaneous process and its values also indicate that the inhibition process becomes less favorable as the temperature is increased from 20 °C to 50 °C. The positive values of adsorptive equilibrium constant (k) reveal a better adsorption, which leads to an increase in the inhibition efficiency [18]. The negative values of ΔG0ads also suggest a strong interaction of the inhibitor molecule on the carbon steel surface [19]. These negative values indicate that adsorption of ADHP on the steel surface is an exothermic process [20].
Generally, an exothermic process signifies either physi-or chemisorption. The heat of physical adsorption is relatively low, on the order of 4-41 kJ.mol-1 , whereas that of chemisorption is much higher, of a magnitude of 100-500 kJ.mol-1 [21]. These concepts can be made valid for the adsorption of ADHP onto the steel surface, whereby it can be affirmed that this is due to a physical phenomenon.
Using Eq.(9) we obtained negative (ΔS0ads) values (see Table 3). The negative steel surface, whereby it can be affirmed that this is due to a physical phenomenon.
values of ΔS0ads in the absence and presence of the inhibitors imply that the activated complex is the rate determining step and represents association rather than dissociation [22]. It also reveals that an increase in the order takes place going from reactants to the activated complex.
Kinetic parameters
The activation energy (Ea) of the corrosion process was calculated using Arrhenius equation [23]
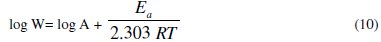
where Ea represents the apparent activation energy, R the gas constant, T the temperature, A the pre-exponential factor, and W is the corrosion rate. Fig. 4 represents Arrhenius plot (log Rcorr vs. (1/T)) for uninhibited and inhibited 1 M HCl containing different concentrations of ADHP.
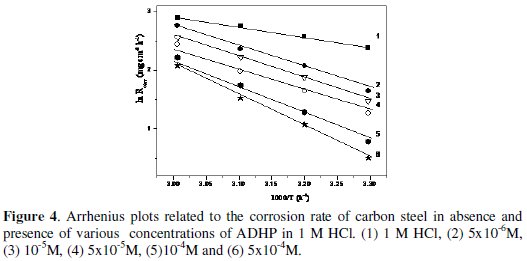
The values of Ea can be obtained from the slope of the straight lines and are listed in Table 4.
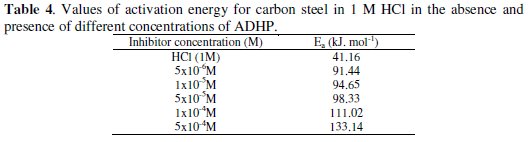
The increase of the activation energy in the presence of the inhibitor is attributed to an appreciable decrease in the adsorption process of the inhibitors on the metal surface with increase of temperature and a corresponding increase in the reaction rate because of the greater area of the metal that is exposed to the acid. A decrease in inhibition efficiency with rise in temperature, with analogous increase in corrosion activation energy in the presence of inhibitor compared to its absence, is a good evidence of the physical adsorption of ADHP on the steel surface.
Galvanostatic polarization studies
The effect of addition of ADHP on the galvanostatic polarization curves of 1018 C-steel in 1 M HCl solution was studied. The effect of increased concentration of ADHP is represented in Fig. 5.
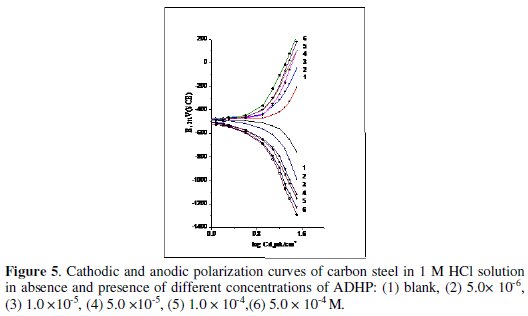
The electrochemical parameters such as, anodic Tafel slope (ba), cathodic Tafel slope (bC), corrosion potential (Ecorr.), corrosion current density (Icorr), and inhibition efficiency (I.E.) were calculated and given in Table 5.
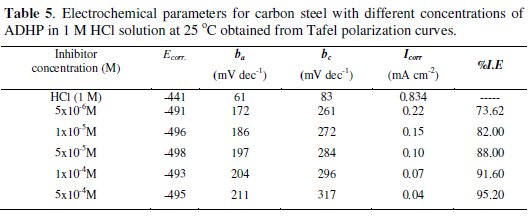
Inspection of the data given in this table reveals that, as the concentration of the inhibitor increases, the values of ba and bc increase. This indicates that the inhibitor affects both anodic and cathodic reactions i.e mixed inhibitor [24]. The values of Ecorr are shifted to more negative potentials, the values of Icorr decrease and the values of IE increases indicating the inhibiting effect of these compounds.
Mechanism of inhibition
The inhibiting effect of ADHP toward the corrosion of 1018 C-steel in 1 M HCl solution could be attributed to several factors including the structure, the number and types of adsorption sites, the nature of molecule, the metal surface, and the ability to form complexes [25].The inhibition action of ADHP is explained due to the formation of complex between Fe2+ and ADHP (Fig. 6).
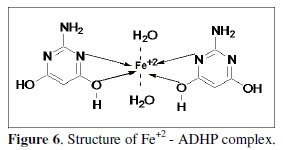
The formed complex is adsorbed on the metal surface and thereby isolating the metal from corroding attack.
Conclusions
1-The corrosion of 1018 carbon steel in 1 M HCl is inhibited by 2-amino-4,6dihydroxy pyrimidine(ADHP).
2-The inhibition efficiency was found to increase by increasing the inhibitor concentrations, and/or decreasing the temperature.
3-The inhibitive effect of these compounds takes place through the adsorption of their compounds on the metal surface.
4-The adsorption process follows Langmuir adsorption isotherm.
5-The inhibition efficiency obtained from weight loss measurements showed good agreement with those obtained from galvanostatic polarization measurements.
References
1. Sykes JM. Br Corros J. 1990:25:175. [ Links ]
2. Abdallah M, Asghar BH, Zaafarany I, Fouda AS. Int J Electrochem Sci. 2012;7:282. [ Links ]
3. Abdallah M, Atwa ShT, Abdallah NM et al. Anti Corrosion Methods Materials 2011;58:31. [ Links ]
4. Hosseini SMA, Salari M, Jamalizadeh E et al. Mater Chem Phys. 2010;119:100. [ Links ]
5. Abdallah M, Megahed HE, Motae MS. Mater Chem Phys. 2009;118:111 [ Links ]
6. Abdallah M, Helal EA, Fouda AS. Corros Sci. 2006;48:1639. [ Links ]
7. Li X, Deng S, Fu H. Corros Sci. 2011;53:3241. [ Links ]
8. Jacob KS, Parameswaran G. Corros Sci. 2010;52:224. [ Links ]
9. Abdallah M, Zaafarany I, Khairou KS, Sobhi M. Int J Electrochem Sci. 2012;7:1564. [ Links ]
10. Abdallah M, El-Etre AY, Soliman MG, Mabrouk EM. Anti Corros Methods Materials. 2006;35:118. [ Links ]
11. Tizpar A, Ghasemi Z. Applied Surf Sci. 2006;252:8630. [ Links ]
12. Abdallah M, Fouda AS, Shama SA, Afifi EA. African J Pure Appl Chem. 2008;2:83. [ Links ]
13. Scendo M. Corros Sci. 2007;49:373. [ Links ]
14. Tebbji K, Bouabdellah I, Aouniti A et al. Mater Lett. 2007;61:79. [ Links ]
15. Oguzie EE, Li Y, Wang FH. J Colloid Interf Sci. 2007;310:90. [ Links ]
16. Makhlouf MTh, Wahdan MW. Polish J Chem. 1995;69:1072. [ Links ]
17. Elachouri M, Haiji MS. Corrosion. 1996;52:103. [ Links ]
18. Talatl JD, Gundhi DK. Corros Sci. 1983;23:1315. [ Links ]
19. Mu G, Li X, Qu Q, Zhou J. Corros Sci. 2006;48:445. [ Links ]
20. Gomma MK, Wadan MH. Mater Chem Phys. 1995;39:209. [ Links ]
21. Popova A, Sokolova E, Raicheva S, Christov M. Corros Sci. 2003;45:33. [ Links ]
22. Abdallah M. Corros Sci. 2004;46:1981. [ Links ]
23. Putilova IN, Balezin SA, Barannik UP. Metallic corrosion inhibitors. New York: Pergamon Press; 1960. [ Links ]
24. Abdallah M. Corros Sci. 2002;44:717. [ Links ]
25. Fouda AS, Mousa M, Taha F, El-Neanaa A. Corros Sci. 1986;26:719. [ Links ]
*Corresponding author. E-mail address: helmymegahed@yahoo.comm
Received 22 December 2012; accepted 31 December 2012














