Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.33 no.5 Coimbra set. 2015
https://doi.org/10.4152/pea.201505249
Effects of Process Parameters on Microhardness of Electrodeposited Ni-Al Composite Coating Using Taguchi Method
S. Jeyaraja,* , K.P. Arulshrib and P.S. Sivasakthivela
a School of Mechanical Engineering, SASTRA University, Thanjavur 613401, TN, India
b Bannari Amman Institute of Technology, Sathyamangalam 638 401,TN, India
Abstract
This paper aims at investigating the influence of process parameters on micro hardness of metal matrix / material particle coated steel plates. The electro deposition parameters such as current density, pH of bath, bath temperature, Al particles concentration in the bath and agitation speed were considered for this study. Nickel matrix / aluminum particle composite coatings were prepared from a Watt's bath by electro-codeposition method. The Taguchi method was used to establish the relationship between the process parameter and response variable, micro hardness of the coated plate. L27 Taguchi orthogonal design was employed for conducting the experiments. The micro-hardness of the deposits was examined using a Vickers micro-hardness tester with the payload of 100 g for 10 sec of indentation period. Signal-to-noise ratio and analysis of variance were employed to determine the significance of the process parameter. The surface morphologies of coating and vol. % of Al particles in deposits were analyzed using optical and scanning electron microscope observations.
Keywords: Ni-Al composite coating; Orthogonal Array; Micro hardness; Taguchi's approach; S-N ratio.
Introduction
Electro deposition is the most convenient technique for fabrication of composite coatings. It has numerous benefits like low cost of fabrication, stumpy energy requirements, accurately controlled process and ability to generate a coating for intricate surfaces and parts [1]. The reinforcing elements are implanted in metal matrix through electro deposition and the composite coatings are formed. The reinforcing elements embedded in the composite coating contain ceramic particles such as SiC, Al2O3, Si3N4, TiN, TiO2 and ZrO2 [2-6], metal particles such as Ti, V, Mo, Cu, Cr, W and Al [7-9], diamond particles [8], PTFE [9], pumice [10] and carbon fibers [11]. Composite coating consists of metal matrix and second phase particles possessed with enhanced mechanical properties such as high hardness, anti corrosion, anti oxidation, tribological characteristics and thermal stability. A new technique has been developed recently in electrodeposition to deposit metal particles in a metal matrix to generate an electrodeposited metal matrix / metal particle composites (EMMCs). The methodology for the development of EMMCs was similar to the conventional type electrodeposition process. The effects of deposition were influenced by process conditions such as current applied, pH of the bath, particle concentrations, bath temperature and agitation of the bath.
Adequate investigations had been done by numerous researchers to investigate the effects of process parameters using conventional methods. Haifeng Liu et al. [12] had investigated the influence of process parameters such as particle loading, On/Off time of string and current density on vol.% of Al particle in Ni- Al composites. They reported that the volume % of Al particles in Ni matrix was influenced by loading of particles up to 40 g/L. Daemi et al. [13] had prepared the Ni-Al composite coating from Watt's nickel bath. They determined that the increase in Al particulates in bath effects with growing trend for adsorbed particles to reach the cathode plate. Also they concluded that the weight percent of Al particles in the coated films augmented with increase of current density. Ghanbari and Mahboubi [14] had reported that the improvement of Vickers micro hardness of Ni-Al particles composite coating was controlled by the plating factors such as current density and particle concentration. Zhou et al. [15] deposited the micro and nano level Al particles in Ni matrix using the electro deposition process to investigate the effect of particle size. They established that the nano sized Al particles significantly promoted the number of particles per unit volume in the composite matrix. The formations of more homogeneous and greater equaled grain structure were observed. Subramanian et al. developed a prediction model for copper content by neural network techniques in bronze electrodeposition [16]. K. Ramanathan et al. conducted their experiments for Ni-Diamond composite coating through design of experiments (DOE) approach by considering the parameters current density, pH and temperature of the bath. They also developed a prediction model for volume fraction of diamond particles in deposit by ANN and regression modeling [17]. NapÅoszek et al. [18] prepared the composites Ni+Ti, Ni+Al and Ni+Ti+Al coatings by electrodeposition technique and found that the deposits produced uniform implantation of Al and Ti particles in Ni matrix. They also established that the metal powder amount and current density were taken as the foremost part in particle deposition into the matrix. Many of these investigations were mainly focused on parameters such as current applied, pH of the bath, particle concentrations, bath temperature and agitation of the bath for preparation of the electrodeposited composite coating.
Design of experiments
Design of experiments (DOE) approach is implemented in several engineering applications to minimize cost and time. This technique can characterize and investigate all the process conditions and factors involved in the experimental work. In DOE, experimental consequences are analyzed by statistical techniques and the significance of process parameters on experimental outcomes can be estimated [19]. Sheng-Lung Kuo [20] reported various statistical techniques that are available to observe the influences of process variables in DOE such as one, fractional, and full factorial; and robust design of experiments. Further the author also described that better quality of the experimental procedure is the primary principle of the robust design method and minimizes the cause variation effects without compromising the roots. Statistical prediction models for various performances in terms of process parameters had been explored by many researchers and also determined the best levels of the process parameters. Aruna et al. [21] investigated the influences of process parameters in preparation of Ni- YSZ composite coatings by L9 orthogonal array of Taguchi's design and S/N ratio analysis. They considered current density, particle concentration and time of deposition on area fraction of YSZ particles, micro hardness and thickness of Ni- YSZ composite coating. They evaluated the mean S/N ratios for experimental outcomes and ranked the influences of process parameters by order. Their prediction models also are in good agreement with the experimental results. The dry sliding wear behavior of in situ casted AA7075-TiC metal matrix composites were investigated by Baskaran et al. [22] by using L27 orthogonal array of Taguchi technique. They took the reinforcement parameters of TiC (wt.%) load(N), sliding velocity (m/s), sliding distance (m) on wear rate. By means of ANOVA analysis they concluded that the load and sliding velocity parameters were highly significant on the wear rate. Statistical prediction models have been applied in various field investigations to evaluate the best levels of the process parameters by many researchers [23-25].
However, previous experimental investigations in composite coatings were conducted by randomized manner. Selection of process parameters also has not been done in proper categorization. In this article, the main aim is to analyze the influence of process parameters such as current density, pH of bath, bath temperature, Al particles concentration in the bath and agitation speed on micro hardness of coatings. Taguchi method has been employed to study the influences of these parameters. Analysis of Variance (ANOVA) technique has been applied to determine the significance of these parameters.
Taguchi method
Taguchi method is an experimental design technique used for engineering analysis to optimize the levels of process parameters for the required performance characteristic. A large number of experiments have to be carried out to study the characteristics of the response influenced by various process conditions. This technique reduces the number of experiments by introducing a special design of orthogonal arrays. The orthogonal array approach helps to study the entire parameter space with minimum number of experiments. Thus, it reduces time and cost of the experiment. Taguchi uses loss function to determine the performance characteristic deviating from the desired value. The loss function value is transformed into signal-to-noise (S/N) ratio. The term ''signal'' represents the desirable (mean) values, and the term ''noise'' represents the undesirable (standard deviation (SD)) values for the output characteristic. Three types of S/N ratio are available based on the output characteristic: lower is better, nominal is better and higher is better. Orthogonal array approach and S/N ratio are the major tools accessible in the experimental design to endorse the accuracy of the experimental work and also to minimize experiments.
In the present work the objective is to maximize the micro hardness of the composite coatings, hence the higher is the better is adapted. The higher is better characteristic S/N ratio can be formulated as
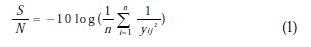
where n is equal to replication of the experimental work and y represents the output of the experiment. In addition to S/N ratio, a statistical technique, ANOVA can be employed to determine the influence of the process parameters on the performance characteristic.
Hence, the above mentioned aspects are the motivations for this study to investigate the influence of process parameters on micro hardness of Ni-Al particle composite coating using Taguchi's orthogonal array studies. L27 orthogonal was employed for conducting the experiment. The process parameters and their levels are shown in Table 1
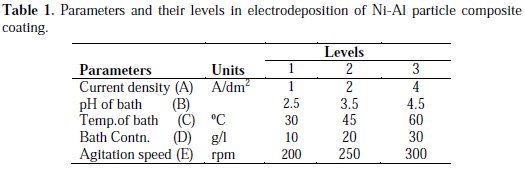
The range of process parameters is limited by conducting trail runs.
Experimental setup
Equipments and materials used
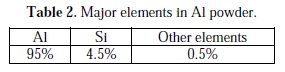
Ni-Al composite coatings were prepared by electrodeposition from Watts's bath [3] solution. The electrolyte medium has been prepared from laboratory graded materials and sterilized by conventional method. 1000 mL of solution were taken for plating work in a 2000 mL BOROSIL glass beaker and the pH of the solution was initially attuned to 5. A pure nickel plate of size (102×43 ×5) mm3 was used as anode for Ni metal matrix formation. A cold rolled mild steel plate of size (71 × 25.4 × 1.2) mm3 was employed as cathode substrate. The cathode plate was degreased with acetone solution and polished using dry cloth buffing wheel, for removal of rust layer. The mass of each mild steel plate was weighed before plating using an electronic balance. The effective area of deposition was taken as (25.4× 25.4) mm2 for plating work on polished surface and the remaining portions of the cathode plate were masked. Fine particles of Al powder with average size of 5 μm with the required quantity were added to the solution. Table 2 shows the constituents of Al powder.
The plating solution along with Al powder was stirred for 3 hours before plating for getting of homogeneous blend along with the surfactant. Each mild steel cathode plate was etched in alkaline bath by electro cleaning for removal oxide contamination in the plating area and to confirm better adhesion of the coating. After alkaline cleaning the cathode plate was rinsed in distilled water and kept immersed in the plating bath.
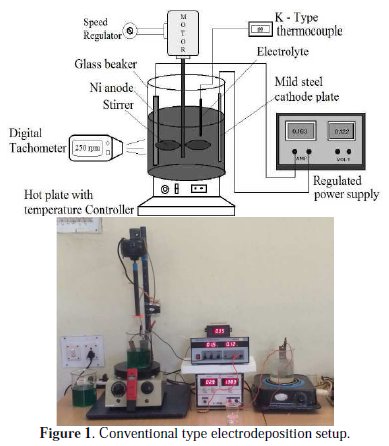
The Al particles were kept in suspension by mechanical agitation using a motorized stirrer. The speed of the stirrer was monitored by a digital tachometer and attuned with a speed controller. A D.C. power source was employed for plating work. A regulated D.C. power supply unit (made by Spark Tek, India, Capacity: 0 - 30 V and 0-2 A) was employed for electrodeposition. A hot plate with a temperature controller unit (made by Osham, India, Capacity: AC type, 230 Volt, 50 Hz, Temperature range: 30 °C to 110 °C) was equipped to heating up of bath to required temperature levels. A 'K'-type thermocouple was employed to monitor the temperature of the bath during plating. The bath pH was observed by a digital type pH meter (made by Hanna, Mauritius) and adjusted to the required level before the commencement of every plating. The pH value of the bath was adjusted by using diluted H2SO4 or else NaOH solutions. The distance between the Ni anode and the mild steel cathode was maintained constant. Both plates were vertically positioned for all experiments. This plating technique is called conventional type electrodeposition (CED) technique [26]. The typical CED type electroplating setup is shown in Fig. 1.
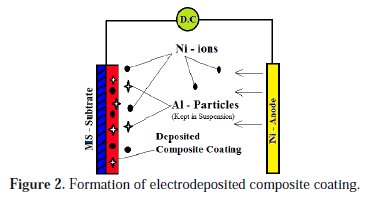
Formation of Ni - Al particles composite coating
The principle of electro co-deposition is similar to the basics of electroplating technique. Addition of reinforcing elements such as ceramic or metal powder into the electrolytic bath is the major task in electro co-deposition. In this study, Al particles were blended in a Watts nickel bath and kept in suspension by mechanical agitation. During plating the charged nickel ion particles which released from Ni-anode captured the Al particles kept in suspension in the bath and deposit the same into the cathode substrate. The continued occurrence of this phenomenon guides the formation of metal matrix composites. Fig. 2 shows the formation of an electrodeposited composite coating.
Experimental design and run orders
The Ni - Al composite coating experimental design and trial runs were created by the Taguchi's method of design of experiments. In this investigation, an L27 orthogonal array was selected for experimental design based on the five parameters and its levels are given in Table 1. The run orders of parameter levels are shown in Table 3.
The experiments were conducted based on the run orders of L27 orthogonal arrays. The plating parameters were precisely controlled during deposition. The time extent for each plating was taken as 60 min for all cases.
Assessment of surface morphology and volume fraction of Al particles
The coated samples were rinsed in distilled water and prepared for surface morphological investigations via metallographic procedures. At first, the orientations and distributions of Al particles in nickel matrix were absorbed using a high transmission Trinocular metallurgical microscope (KYOWA, model MELUX2, 50x-1000x, Japan) with different magnifications. The area and volume fractions of Al particles in the matrix were examined via CCD camera (WATEC, model WAT-221S, Japan) and image analyzer system. Fig. 3 shows the distribution of Al particles in nickel matrix captured in the optical CCD camera.

Surface morphologies of Ni-Al particle coatings were observed with a Scanning Electron Microscope (JEOL-Field emission SEM, model TSM-6701F, Japan) with various magnifications and are shown in Fig. 4.

The observed results are manipulated and noted in Table 3.
Assessment of micro hardness (HV) of Ni-Al coating
The micro hardness of the coated sample was investigated in a Vickers micro hardness tester (model & maker: SHIMADZU -TYPE HMV-1/-2, SHIMADZU Corporation, Japan) with the payload of 150 gm for 10 sec of indentation period. The indented location was focused at 400× magnification and the slider position was attuned to the diagonal lengths of indentation. The micro hardness was calculated by a system based on and value was taken from digital read out. Micro hardness of each sample was inspected with three trials and the average value was manipulated. The average values of micro hardness observed are tabulated in the Table 3.
Results and discussions
The experiments were conducted based on the run orders of L27 orthogonal array through adjusting the process parameter and levels. Because of the presence of constant coulomb in the circuit, consistent deposition was achieved in the coatings. Coating thickness of the deposits was attained in deposits about 21 to 52 μm. Al particles were embedded in Ni matrix with various volume fractions. The mass of deposition gained from deposits was about 12.7 to 584.6 mg. The span of volume fractions of Al deposition was laid between 7.35 to 21.14 %. The micro hardness that was found in the deposits varied from 146 to 306 HV. The influences of process parameters on micro hardness of the deposits were analyzed by mean effect studies of S-N ratio analysis. The significance of the process parameters was determined using ANOVA technique. The effects of plating parameters on micro hardness were studied systematically and the results were discussed underneath.
Analysis of S/N ratio
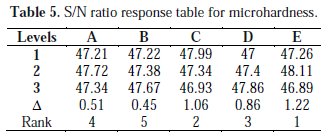
We need to investigate the S/N ratio factor from the experimental data sets to compute the average S/N ratio response for each experimental factor. From the mean S/N response factor, the most favorable plating conditions for each design parameters can be identified and the design (plating) parameters can be ranked according to their impact on the response parameter. In this experimental design, micro hardness of the deposit is the response variable which needs to be maximized and hence larger the better characteristics was chosen for this experimental investigations. After manipulation of the S/N ratio for experiment trails, the average S/N ratio value was calculated for each factor and level. The experiment outcomes and S/N ratio values are given in Table 3.
The mean micro hardness response table for each level of process parameters was created in the integrated manner. The average value of micro hardness for each parameter at each level was calculated and is shown in the Table 4.
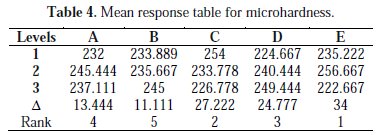
Table 4 indicates the mean of the response variable (micro hardness) for each level of each control factor. The same procedure is applied for S/N ratio response for each level of the process parameter, and the S/N ratio response for micro hardness is given in Table 5.
From Table 4, based on the mean value of the micro hardness for each level, the difference between the maximum and minimum values was calculated. The maximum difference will give the most significant parameters, and the rank for the significant parameters is depicted. From Table 4, it is inferred that the optimal combination that yield maximum micro hardness of deposits is A2 B3 C1 D3 E2. The ranks of the significant parameters are rated as Agitation speed (rank 1), Temperature of bath (rank 2), Bath concentration (rank 3), current density (rank 4) and pH of bath (rank 5). The effect of process parameters resulting from the optimization process is plotted in Fig. 5.
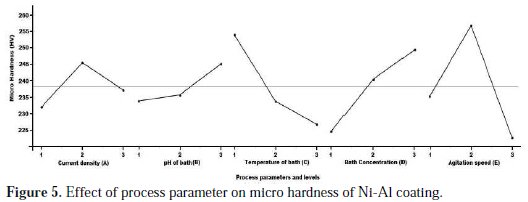
The results of mean S/N ratio response values are shown in Fig. 5. This table also includes delta (Δ) which is the difference among the highest S/N ratio and the lowest S/N ratio values. Ranks for factors are allocated on the basis of the delta value. The highest delta value is assigned to rank 1; rank 2 is assigned to next highest delta value and the rest. Based on ranking positions, it was observed that the agitation speed has the highest delta value, ranked by 1 and identified as the most influencing factor on micro hardness. The other factors such as temperature, bath concentration, current density and pH factor were ranked by order. The average factor effect with S/N ratio is shown in Fig. 6.
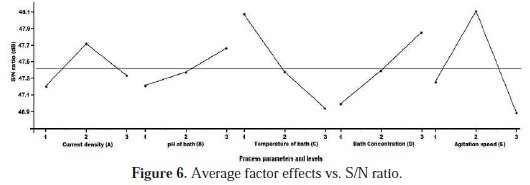
This plot illustrates the effects of factor levels with S/N ratio.
ANOVA
ANOVA, a statistically based objective decision making tool, was employed to examine the influence of process parameters on quality characteristics. It helps in testing the significance of all process parameters by comparing the mean square against an estimate of the experimental error at specific confidence levels. This is done by calculating the variability of the S/N ratios (sum of the squared deviations from the total mean S/N ratio) into contributions by each process parameter and error. The percentage contributions of variance are estimated by the following equations. The total sum of the squared deviations (SST) from the total mean S/N ratio can be expressed as
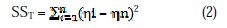
where 'n' is the number of experiment in the orthogonal array, 'ηi' is the S/N ratio of the ith experiment and 'ηn' is the total mean S/N ratio. The percentage contribution of variance (Ï) can be calculated as follows;
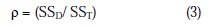
where SSD is the sum of the squares of deviation. F-test is a statistical tool (the mean square error to residual) in ANOVA used to determine most significant process parameters that influence the quality characteristic. Higher the F-value will be, most influent on the response quality characteristic. P-value demonstrates the significance level (significant or non significant) of the process parameter.
Table 6 gives the results of ANOVA for micro hardness.
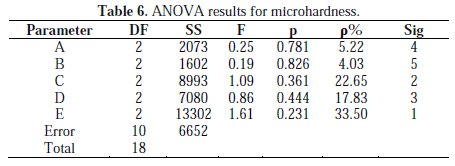
It is observed that the most significant parameters that influence microhardness of the coating are order of agitation speed, E (33.5%); temperature of bath, C (22.65 %); bath concentration, D (17.83%); current density, A (4.9%) and pH of bath, B (4.24%).
Influence of process parameters on Micro hardness
Influence of current density
The effect of current density on micro hardness of deposit is shown in Fig. 7.
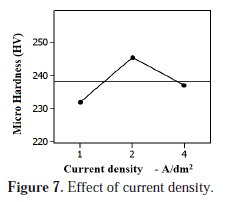
During electro-codeposition, the Al particles are resided into the space available in the matrix along with metal ions. The plot illustrates that the micro hardness of the deposit was in a deteriorated condition at 1 A/dm2. Due to the weaker magnitude level of the current density, the deposition rate of Ni-Al particles was reduced at lower current density level. On the other hand, better micro hardness was obtained at 2 A/dm2 current levels. In this level the deposition rate was amplified due to the moderate magnitude level of the current density which supports enhanced deposition and micro hardness. Many researchers [3, 27] have obtained a better deposition of reinforcement elements in matrix and micro hardness at 2 A/dm2. Further increases in current density have distressed the deposition and micro hardness of the coating. The micro hardness of the deposit was abridged at 4A/dm2. At this level the deposition rate was not sustained due to erratic current magnitudes which lead decrease in micro hardness. Numerous investigators have explored that the higher current density levels are not suitable for better deposition and end properties.
Influence of pH
Fig. 8 shows that the pH of the bath has an adverse effect at lower levels content.
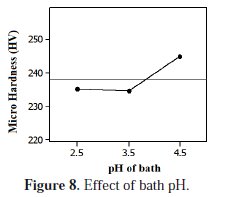
The micro hardness of the deposit was decrease up to pH 3.5 and then increases. Narasimman et al. and Lee et al. investigated that the co-deposition behavior of reinforcing particles was increased at pH 4. Also the investigators have accredited that this phenomenon is owed to the modification in zeta potential which is influenced by the pH factor of the bath and ionic force of the plating medium [10]. The greater micro hardness of the coating was achieved in pH 4.5 levels. These observations have described lower pH circumstances not suitable for composite plating with better micro hardness.
Influence of bath temperature
The average effect of the bath temperature on the micro hardness of the coating is presented in Fig. 9.
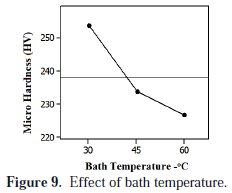
In the current study, the experiments were conducted between 30 °C to 60 °C of bath temperature. In general the nickel bath is operated between 40-60 °C for composite plating. To recognize the effect of room temperature, the current experiments were started with 30 °C. It has been observed that the improved deposition rate and micro hardness of the coating was recovered between 30 °C to 45 °C. It was found that the micro hardness of the coating was decreased at higher bath temperature conditions. The increase in bath temperature caused the enhanced ionic transportation [3] which affects the end properties in deposition.
Influence of Al particle concentration in the bath
The amount of co-deposition of Al particles in the matrices was augmented with increase in concentration of particles in the plating bath. The Fig. 10 shows the intensifying trend of micro hardness by means of increase in the bath concentration.
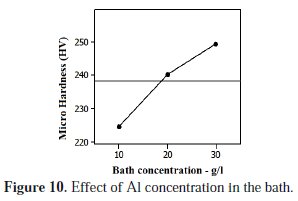
The amplifications in bath concentration have caused the possibility of increased number of Al particles being in movement towards the cathode plate. Those particles were absorbed in the cathode surface for an adequate period of time and effectively included in the growing nickel matrix. Due to these reasons, micro hardness of the matrix was increased in elevated concentration levels. The improved micro hardness values were registered between 20 to 30 g/L concentration levels.
Influence of agitation speed
The co-deposition rate of Al particles was favorable at lower speed levels and then decreased with increase in agitation speed. The average effect of the agitation speed on the micro hardness of coating is shown in Fig. 11.
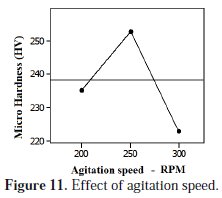
At lower speed levels the particles were in fine stream line suspension levels and the deposition rate was increased. This occurrence has directed the increase in micro hardness. The enhanced micro hardness was obtained between 200-250 rpm levels. Further increase in agitation speed caused the turbulence motion of the particles and affects the particle adhesion in the matrix. Also the unrest particles have disturbed the settled particles in the matrix. Thus the deposition rate of Al particles and micro hardness were decreased at higher speed levels. It is concluded that the lower agitation speed levels have offered the better micro hardness assessments.
Conclusions
The micro hardness of metal matrix / material particle coated steel plates was measured and the surface morphologies of the coating and the vol. % of Al particles in the deposits were analyzed in this study. This paper presented the application of Taguchi method to study the effect of the process parameters on the microhardness of the nickel matrix / aluminum particle composite coatings.
The following conclusions were established from the experimental and analytical studies.
The Taguchi's approach L27 orthogonal array methodology was implemented to study the effects of five primary parameters of the electrodeposited Ni - Al particle composite coatings. The effect of the process parameters on the microhardness was evaluated using Taguchi method. The agitation speed was found to be the most significant parameter that influences the microhardness of the deposit. The optimal combinations of the process parameters for maximum microhardness were determined.
The surface morphologies of the Ni-Al coatings were examined with microscopy and SEM investigations.
The mean S/N ratio values are computed for process parameters and their levels.
The delta values of factors are ranked by highest to lowest. It is recognized that the agitation speed is the most significant factor for the response.
This kind of statistical investigations improves the reliability of an experimental work instead of conventional and randomized testing procedures. It also saves time and cost of experiments.
It is essential to note that a micro hardness of coatings was achieved in the span of 146 to 306 HV and the volume fraction of Al particles was about 7.38 to 21.14%.
References
1. Fei C, Chuanhai J, Xueyan W. J Alloys Comp. 2014;604:292. [ Links ]
2. Saha RK, Khan TI. Surf Coat Technol. 2010;205:890. [ Links ]
3. Narasimman P, Malathy P, Periasamy VM. Appl Surf Sci. 2011;258:590. [ Links ]
4. Fafeng X, Chao L, Chunhua M, et al. Int J Refractory Metals Hard Mater. 2012;35:295. [ Links ]
5. Feng C, Hu S, Jiang Y, et al. Rare Metal Mater Eng. 2013;42:2427. [ Links ]
6. Krishnaveni K, Narayanan TSNS, Seshadri SK. J Alloys Comp. 2008;466:412. [ Links ]
7. Pedro LN, Adriana NC, Renato ACS, et al. Electrochim Acta. 2010;55:2078. [ Links ]
8. Fei C, Chuanhai J. Appl Surf Sci. 2014;292:620. [ Links ]
9. Balaji R, Malathy P, Kumar KY, et al. Surf Coat Technol. 2006;201:3205. [ Links ]
10. Aruna S T, Shibayan R, Amit S, et al. Surf Coat Technol. 2014;251:201. [ Links ]
11. Byung-Joo K, Woong-Ki C, Moon-Kwang Um, et al. Surf Coat Technol. 2011;205:3416. [ Links ]
12. Haifeng L, Weixing C. Surf Coat Technol. 2005;191:341. [ Links ]
13. Daemi N, Mahboubi F, Alimadadi H. Materials Design. 2011;32:971. [ Links ]
14. Ghanbari S, Mahboubi F. Materials Design. 2011;32:1859. [ Links ]
15. Zhou Y B, Qian B Y, Zhang H J. Thin Solid Films. 2009;517:3287. [ Links ]
16. Subramanian K, Periasamy VM, Pushpavanam M, et al. Port Electrochim Acta. 2009;27:47. [ Links ]
17. Ramanathan K, Periasamy VM, Natarajan U. Port Electrochim Acta. 2008;26:361. [ Links ]
18. NapÅoszek-Bilnik I, Budniok A, Losiewicz B, et al. Thin Solid Films. 2005;474:146.
19. Benjamin G, Stefan A, Andrey G, et al. Procedia CIRP. 2013;11:245. [ Links ]
20. Sheng-Lung K. J Chinese Inst Eng. 2004;27:243. [ Links ]
21. Aruna S T, Srikanth PVK, Ahamad MJ, et al. Port Electrochim Acta. 2011;29:23. [ Links ]
22. Baskaran S, Anandakrishnan V, Muthukannan D. Materials & Design. 2014;60:184. [ Links ]
23. Manoj M, Rajendrakumar PK. Materials Design. 2011;32:3637. [ Links ]
24. Kalyan DS, Prasanta S. Materials Design. 2011;32:2228. [ Links ]
25. Shravani D, Lakshmi PK, Balasubramaniam J. Acta Pharmac Sinica B. 2011;1:56. [ Links ]
26. Narasimman P, Malathy P, Periasamy VM. Port Electrochim Acta. 2012;30:1. [ Links ]
27. Susan DF, Barmak K, Marder AR. Thin Solid Films. 1997;307:133. [ Links ]
*Corresponding author. E-mail address: sjraj81@gmail.com
Received 9 January 2015; accepted 21 July 2015














