Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Portugaliae Electrochimica Acta
versión impresa ISSN 0872-1904
Port. Electrochim. Acta vol.33 no.5 Coimbra set. 2015
https://doi.org/10.4152/pea.201505305
Inhibition of Lead Corrosion in 0.1 M Na2CO3 by Artemisia Oil
El-Miziani Inaama,b,* , Houbairi Saraa , Essahli Mohameda , Lhaloui Saadiab and Lamiri Abdeslama
a Laboratory of Applied Chemistry and Environment, Faculty of Science and Technology, University Hassan First, Settat-Morocco.
b Laboratory of Entomology, National Institute of Agronomic Research-CRRA, Settat, Morocco.
Abstract
The essential oil of Artemisia herba alba has been extracted and tested as an inhibitor of lead corrosion in 0.1 M Na2CO3 medium, using stationary and transient electrochemical techniques. The results obtained show that the essential oil of Artemisia reduces the corrosion rate. The effectiveness of inhibition increases with the oil concentration to reach 77 % with 2000 ppm. The effect of temperature was also studied.
Keywords: corrosion, inhibition, lead, Artemisia, essential oil, adsorption.
Introduction
The growing need for corrosion inhibitors is becoming increasingly necessary to stop or delay the attack of a metal in an aggressive solution. The major application areas are: acid etching, cleaning with industrial acid, scaling and acidification.
Considerable efforts are made to find suitable compounds for use as corrosion inhibitors in various corrosive environments [1-8].
These organic compounds are either synthesized or extracted from spices and aromatic and/or medicinal plants. Naturally antioxidants, their use as corrosion inhibitors has been preferred, both for their economic value and for the environmental objectives. These benefits have encouraged us to pull a large part of our laboratory program to examine the inhibitory power of different extracts from natural substances. We found that thyme [9-10], castor [11], spearmint [1213], verbena [14], cloves [15], myrtle [16], eucalyptus [17], etc., possess the power to be very effective corrosion inhibitors.
The encouraging results achieved by the oil from Artemisia herba alba as steel corrosion inhibitor in HCl 1M solution [18], as well for copper in HNO3 2M [19], allowed us to expand its use in a basic solution. In fact, Artemisia herba alba, known in arabic such as Chih, is a spontaneous plant which abounds in desert of Middle East, Mediterranean Basin and sub-Saharan high plateau. Artemisia herba alba as aromatic plant is widely exploited for its essential oil. The latter is characterized by an extraordinary chemical polymorphism. The objective of this work is to study by polarization and electrochemical impedance methods, the effect of wormwood white oil on the lead corrosion in a solution of Na2CO3 0.1 M. The effect of temperature was also studied.
Experimental
Extraction of artemisia herba alba essential oil
Artemisia herba alba (Fig. 1) has been collected from the region of Agadir, Morocco.
The aerial parts of the plant were air-dried in the laboratory at ambient temperature. The essential oil was obtained by steam distillation using a Clevenger type distiller for about 3 hours. The essential oil yield of A. herba alba is 1,1%. This essential oil yield has been calculated on the basis of the dry matter.
After extraction, a part of the essential oil was analyzed for its chemical composition by gas chromatography coupled with mass spectrometry technique. The other part was tested for anticorrosive activity. The oil obtained after extraction, was recovered and stored in a dark bottle at 4 °C before use.
Preparation of the solution
The solution of sodium bicarbonate (Sigma-Aldrich) Na2CO3 0.1 M was prepared with distilled water. Test solutions were freshly prepared before each experiment, by adding the oil directly to the corrosive solution. Experiments were conducted in three repetitions to allow determination of reproducibility.
Electrochemistry measurements
The electrochemical experiments were performed in a pyrex cell, equipped with a conventional three electrodes (lead as an electrode working disc cup-shaped with a geometric area of 1 cm2, platinum as auxiliary electrode and a saturated calomel electrode SCE as reference electrode). The lead disk was abraded with sandpaper for different particle size up to 1200, degreased with acetone, rinsed with distilled water and dried before each test. The measurements are carried out with a mounting including a potentiostat-galvanostat PGZ100, type radiometer, associated with the ''voltamaster4'' software.
The current-potential curves are obtained by potentiodynamic mode; the potential applied to the sample varies continuously with a scanning rate of 2 mV/sec, between -1000 and 1000 mV.
The electrochemical impedance spectroscopy measures (EIS) was carried out with similar previous electrochemical system. The frequencies between 100 kHz and 10 Hz were superimposed on the corrosion potential. The impedance diagrams are given in the Nyquist representation.
The chronoamperometric curves were traced to a potential of 150 mV for 3600 seconds.
Results and discussion
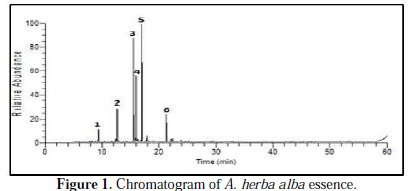
Analysis of artemisia essential oil
The analysis of the chemical composition A. herba alba essential oil shows the presence of chemotype to alpha thujone and camphor. Table 1 contains the main constituents of the essential oil.
Effect of concentration
The potentiodynamic polarization measurements
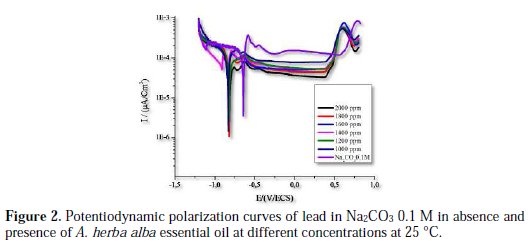
Fig. 2 shows the plots of cathodic and anodic polarization of lead immersed in Na2CO3 0.1 M at 293 K in absence and presence of different concentrations of the inhibitor.
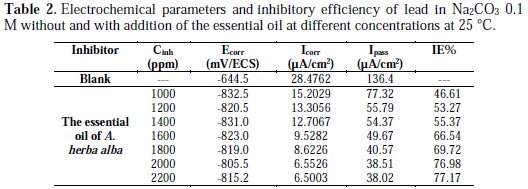
The electrochemical data, corrosion potential (Ecorr), the corrosion current density (Icorr), and passivation (Ipass) are listed in the Table 2.
The inhibition effectiveness (%) is calculated by equation 1:

where Icorr and I'corr represent, respectively, the corrosion current density determined by extrapolation of Tafel straights of the corrosion potential without and with addition of the inhibitor.
The inspection of these results revealed that the corrosion density (Icorr) decreased in the presence of the inhibitor. Indeed, with addition of 2000 ppm of the essential oil, the decrease of the corrosion intensity is noted by a factor of 4.3457; this behavior reflects its ability to inhibit the corrosion of lead in a solution of 0.1 M Na2CO3.
It is eventually clear from Fig. 2 and Table 2 that the adsorption of the inhibitor moves slightly the corrosion potential (Ecorr) in the most negative direction compared to blank, which means that the decrease in cathodic reaction is the main effect of this corrosion inhibitor [20]. Therefore, these results indicate that the essential oil of A. herba alba acts as an inhibitor of mixed type [21]. The increase of passivation current is also noted from a value of 136 μA/cm2 to a value of 38 μA/cm2. Indeed, the addition of 2000 ppm decreases the passivation current Ipass by a factor of 3.5. This decrease is mainly due to the adsorption of oil on the metal surface.
The inhibition efficiency increases with inhibitor concentration to reach 76% at 2000 ppm of A. herba alba essential oil by reducing the density current from a value of 28.47 μA/cm2 to a value of 6.55 μA/cm2.
Chronoamperometry
The chronoamperometric tests of the current as a function of time were performed to identify the type of lead corrosion in sodium bicarbonate solution in the absence and presence of the inhibitor. The chronoamperometric curves of lead measured at 150 mV in Na2CO3 0.1 M without and with more than 2000 ppm of Artemisia herba alba essential oil are presented in Fig. 3.
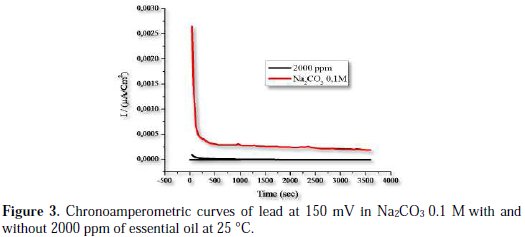
It is clear that higher values of oxidation current of lead dissolution are recorded in Na2CO3 solution without inhibitor; it is due to the corrosive attack of OH- ions. The initial higher values of current occurs because lead dissolves in a first time to form Pb(OH)4, which is unstable thus is transformed to PbO2 when the current decreases with time due to the formation of an oxide layer (PbO2). The formation of such a layer provides a partial protection and doesn't allow current to rise [22]. Adding 2000 ppm of essential oil causes a sharp decrease in the current value, which is probably due to the adsorption of inhibitor on the surface of lead molecules, and the blocking of active sites, thus preventing its dissolution. These results are in good agreement with the polarization data, Fig. 2 and Table 2, and confirm the ability of Artemisia herba alba to inhibit lead corrosion.
The electrochemical impedance spectroscopy measurements
Nyquist performances for lead in Na2CO3 0.1 M solution in absence and presence of different concentrations of Artemisia herba alba essential oil are given in Fig. 4.
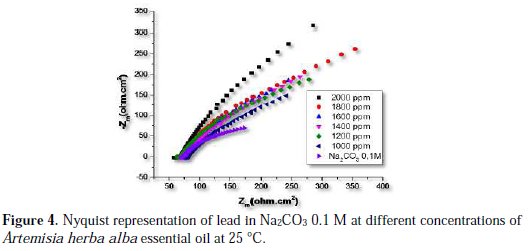
The impedance spectra were simple semicircles, and their diameter increases with the increase of inhibitor concentration. The presence of a single semicircle indicates that the charge transfer occurs at the interface electrode / solution, and the transfer process reaction of lead corrosion in presence of the inhibitor doesn't change its dissolution mechanism [23].
The inhibitory effectiveness was determined by equation 2:
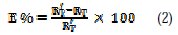
where RT and RT' are respectively the charge transfer resistance of lead in Na2CO3 0.1 M medium in absence and presence of inhibitor. The dielectric parameters are given in Table 3.
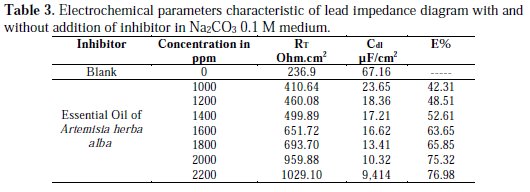
The values of RT' increase with the growth of inhibitor concentration while Cdl values are decrease. The increase of values may suggest the formation of a protective layer on the electrode surface [24].
The charge transfer resistance values (RT) were calculated according to the difference between impedance values at lower and higher frequencies. The Cdl values are considered inferior to those of blank value, which confirms that the adsorption of the inhibitor onto the metal surface forms a double electronic layer [25]. These results are in good agreement with those obtained by polarization where Artemisia herba alba essential oil is proved to be a good inhibitor for lead corrosion in basic medium Na2CO3 0.1 M with an efficiency of 77 %.
Effect of temperature
Temperature is an important parameter that affects the corrosion phenomenon. The measurements are taken at different temperatures (278-308 K), in absence and presence of 2000 ppm of essential oil in 0.1 M Na2CO3.
Polarization measurement
The effect of temperature without and with addition of A. herba alba essential oil, done by polarization measurements, is shown in Fig. 5 and 6, respectively.
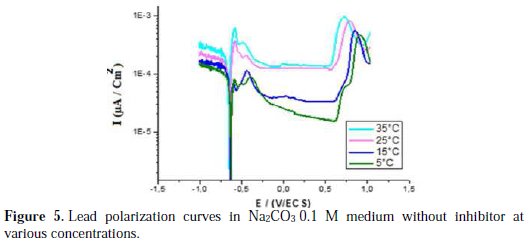
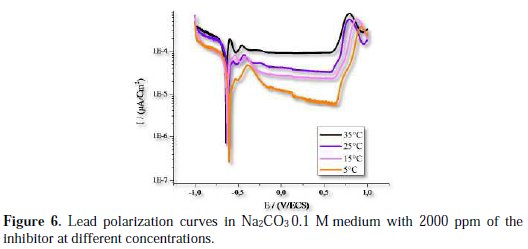
The electrochemical parameters from these tests are given in Table 4.
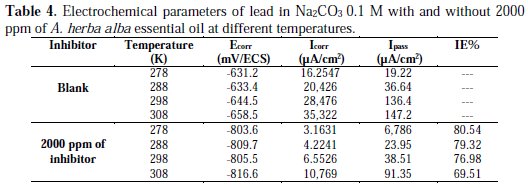
The results in Fig. 5 and 6 and Table 4 indicate that there is a general increase in the intensity of the corrosion within the temperature of 278 to 308 K. The solution becomes more corrosive with rising temperature and the anticorrosive activity becomes less effective. This explains that Artemisia herba alba is adsorbed on the metal by electrostatic bonds (weak bonds). This type of links sensitive to temperature cannot fight effectively against corrosion when the temperature increases [26].
Electrochemical impedance spectroscopy (EIS)
The effect of temperature on lead corrosion behavior in Na2CO3 0.1 M containing a concentration of 2000 ppm of the inhibitor was studied in a temperature range between 278 and 308 K using impedance diagrams (Fig. 7 and 8); the corresponding results are summarized in Table 5.
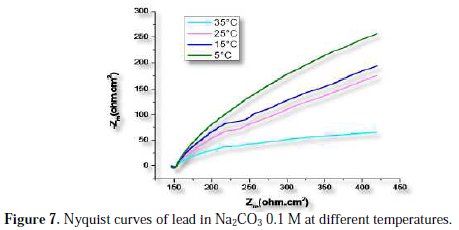
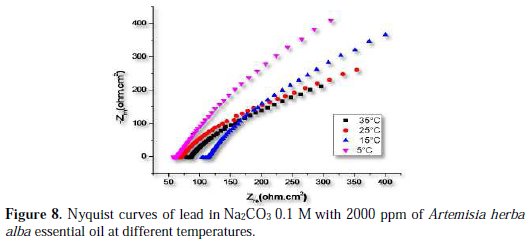
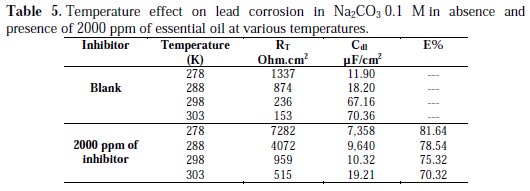
The increase of lead corrosion rate is more pronounced with higher temperature in Na2CO3 0.1 M. The inhibitor effectiveness is affected by temperature; the results show that Artemisia herba alba essential oil inhibitor effectiveness decreases when temperature increases.
Determination of activation energy
To better explain the effect of temperature on the corrosion rate, it is useful to calculate the activation energy value by using the Arrhenius equation and therefore consider that the logarithm of the corrosion rate is a linear function of 1/T. Thus, we can calculate the activation energies from the relations 3 and 4:
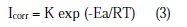
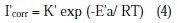
where K and K' are constants (pre-exponential parameter Arrhenius), and Ea and Ea' are the activation energies, respectively, in absence and presence of the inhibitor.
Some conclusions on the mechanism of inhibitors action may be obtained by comparing Ea measured at a time in presence and absence of the corrosion inhibitor. Fig. 9 represents the Arrhenius plot coordinates of lead corrosion rate in Na2CO3 0.1 M with and without essential oil of Artemisia to 2000 ppm.
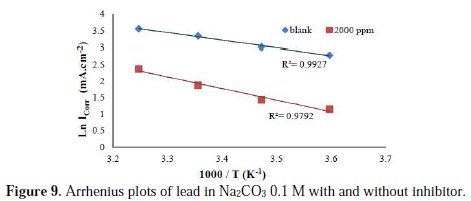
The variation of the logarithm of corrosion current as a function of 1/T gives lines indicating that the Arrhenius law is respected. The values of activation energies obtained from these lines are given in Table 6.

In view of the results of Table 6 it is clear that in presence of the inhibitor the activation energy increases, whereas IE% decreases when the temperature increases. This behavior demonstrates physisorption phenomenon of the inhibitor on metal surface. The recovery rate, low at higher temperatures, suggests that at these temperatures the destruction rate of the film physically adsorbed increases faster than its rate formation.
The values of activation energies obtained from Arrhenius lines are about 18.93 KJ.mol-1, at a concentration 2000 ppm of inhibitor, which means that when the recovery rate is maximum, the value of activation energy in the presence of this oil is 29.16 KJ.mol-1. This confirms the physical adsorption of Artemisia herba alba essential oil by formation of an effective superficial film.
The kinetic parameters, the enthalpy and entropy of the corrosion process are also evaluated from a study of the temperature effect. An alternative formulation of the Arrhenius equation (5) is [27]:
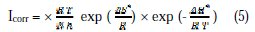
where h is the constant of Planck, N is the number of Avogadro, ΔS* is the entropy of activation and the ΔH* is the enthalpy of activation. Fig. 10 shows plots of Ln (Icorr / T) compared to 1/T. The lines are obtained with a slope of -ΔH*/R and an interception of (LnR/Nh + ΔS*/R), from which the values of ΔS* and ΔH* can be calculated; these values are given in Table 7.
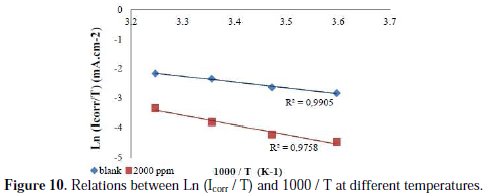

The inspection of these data revealed that the thermodynamic data (ΔS* and ΔH*) for lead dissolution reaction in Na2CO3 0.1 M in presence of the inhibitor is higher than that in its absence. The positive sign of ΔH* reflects the endothermic nature of lead dissolution process, which suggests that lead dissolution is slow in presence of the inhibitor [28]. By comparing the values of activation entropy ΔS* given in the Table 7, it is clear that the activation entropy decreases negatively to a greater extent in presence of white wormwood essential oil, which reflects the formation of a stable layer controlled by the inhibitor on lead surface [28].
Conclusions
The following conclusions can be drawn from this study:
- The Artemisia herba alba essential oil is an effective inhibitor of lead corrosion in a solution of Na2CO3 0.1 M. The inhibition effectiveness increases with the concentration to achieve 77 % to 2000 ppm.
- The results of polarization studies suggest that this oil acts as mixed inhibitor.
- The positive value obtained from the activation energy reveals physisorption.
- The value of ΔH shows that lead dissolution is endothermic.
References
1. El-Etre A Y, Abdallah M, El-Tantawy Z E. Corros Sci. 2005;47:385. [ Links ]
2. Orubite K O, Oforka N C. Mater Let. 2004;58:1768. [ Links ]
3. El-Etre A Y. Corros Sci. 2003;45:2485. [ Links ]
4. Avwiri G O, Igho F O. Mater Let. 2001;57:3705. [ Links ]
5. El-Etre AY, Abdallah M. Corros Sci. 2000;42:731. [ Links ]
6. Al-Sehaibani H. MaterWissenWerkst Tech. 2000;31:1060. [ Links ]
7. Martinez S, Stern I. Appl Surf Sci. 2002;199:83. [ Links ]
8. Houbairi S, Essahli M, Lamiri A. Port Electrochim Acta. 2015;33:305. [ Links ]
9. Houbairi S, Essahli M, Lamiri A. Int J Eng Res Tech. 2014;3:43. [ Links ]
10. Houbairi S, Lamiri A, Essahli M. Int J Eng Res Tech. 2014;3:110. [ Links ]
11. Bensabah F, Houbairi S, Essahli M, et al. Port Electrochim Acta. 2013;31:195. [ Links ]
12. Elmiziani I, Essahli M, Lamiri A, et al. Chem Sci Rev Lett. 2014;3:759. [ Links ]
13. Bensabah F, Houbairi S, Essahli M, et al. J Adv Chem. 2014. [ Links ]
14. Houbairi S, Lamiri A, Essahli M. Chem Sci Rev Lett. 2014;3:353. [ Links ]
15. Houbairi S, Souad R, Lamiri A, et al. Chem Sci Rev Lett. 2015;4:360. [ Links ]
16. Elmiziani I, Houbairi S, Essahli M, et al. IJEIT. 2014;3. [ Links ]
17. Bouyanzer A, Hammouti B. Pigment Resin Technol. 2004;33:287. [ Links ]
18. Houbairi S, Essahli M, Lamiri A. Int J Eng Res Tech. 2014;4. [ Links ]
19. Hasanov R, Bilge S, Bilgic S, et al. Corros. Sci. 2010;52:984. [ Links ]
20. Okafor P C, Zheng Y. Corros Sci. 2009;51:850. [ Links ]
21. Hammouti B, Benayada A, El Maslout A. Bull. Electrochem. 2000;16:283. [ Links ]
22. Li X, Deng S, Fu H. Corros Sci. 2010;52:2786. [ Links ]
23. Behpour M, Ghoreishi S M, Mohammadi N, et al. Corros. Sci. 2010;52:4046. [ Links ]
24. Kumar K P V, Pillai M S N, Thusnavis G R. Port Electrochim Acta. 2010;28:373. [ Links ]
25. Fouda AS, Mohamed AK. J Electrochem Soc India. 1990;39:244. [ Links ]
26. Bochris J O M, Reddy AKN. Modern Electrochemistry. Vol 2. New York: Plenum Press; 1977. [ Links ]
27. Guan N M, Xueming L, Fei L. Mater Chem Phys. 2004;86:59. [ Links ]
28. Yurt A, Balaban A, Kandemir SU, et al. Mater Chem Phys. 2004;85:420. [ Links ]
*Corresponding author. E-mail address: inaam30@hotmail.fr
Received 20 January 2015; accepted 13 October 2015














