Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Portugaliae Electrochimica Acta
versión impresa ISSN 0872-1904
Port. Electrochim. Acta vol.34 no.2 Coimbra mar. 2016
https://doi.org/10.4152/pea.201602097
Voltammetric characterization of grafted polymer electrode self modification with carbon nanotubes (GPESMCNT)
Muhammed M. Radhia* and Emad A. J. Al-Mullab
Middle Technical University, Health and Medical Technology College-Baghdad, Iraqb Department of Chemistry, Faculty of Science, University of Kufa, P.O. Box 21, An-Najaf 54001, Iraq
Abstract
A novel self modification of grafted polymer working electrode with carbon nanotubes was success for fabrication from grafting polymer via gamma irradiation and ferrous ammonium sulfate (FAS) as a catalyst. The electrochemical properties of the self modified grafted polymer with CNT (GPESMCNT) improved performance the working electrode at higher conducting surface was done through using in cyclic voltammetry (CV). The GPESMCNT was characterized by surface analytical methods including AFM and SEM. The characterization of electrocnductivity properties of GPESMCNT was studied in 1M of KCl with different concentration of K3[Fe(CN)6], at different scan rates, temperature, and different concentrations using CV technique. The new GPESMCNT improved performance the working electrode in CV at different techniques such as rotating disc electrode (RDE). also, the nanomaterials in the chain of grafted polymer was enhanced the redox current peaks of Fe(II)/Fe(III) multi times than at commercial working electrodes such as GCE, Pt-electrode, Au-electrode, etc.
Keywords: grafted polymer electrode self modified, CNT, cyclic voltammetry, K3[Fe(CN)6].
Introduction
The modification of grafted polymer with nano-deposits such as CNT, C60 and activated carbon is very important for the scientists esp. in the electrochemistry by cyclic voltammetric analysis field [1-5].
The unique chemical, physical, electronic (metallic or semiconducting) and high thermal properties of carbon nanotubes (CNTs) made them interesting materials for widespread application in the fields such as electrochemical sensors, biosensors, supports for heterogeneous metal catalysts in organic synthesis, fuel cells, semiconductors, batteries, random access memory cells, field effect transistor, field emission display, atomic force microscopy probes, microelectrodes, specific adsorbents to remove organic pollutants from water and waste water and as a potential drug carriers in cancer therapy[6-9]. Working electrodes must be an electronic conductor and electrochemically inert. Commonly used working solid electrode materials for cyclic voltammetry include platinum, gold and glassy carbon. Other materials (e.g., semi-conductors, for example ITO, indium-tin oxide, or conductive polymers or grafted polymer) are also used, for more specific applications [10,11].
Electrochemical behaviour of famotidine has been studied at composite polymer membrane working electrode. Cyclic voltammetric method has been developed for the determination of drug in pharmaceutical formulation. A well-defined anodic peak was observed for famotidine in the entire pH range. The current increases steadily with scan rate and concentration. This composite film showed good catalytic behaviour, which includes a good current response. The result is compared with the glassy carbon electrode and it was found that the current with composite polymer electrode is of the order of 18.60 mA whereas with glassy carbon electrode it was around 565.00 μA [12].
Electrochemical study behavior of terthiophene and its corresponding polymer, which is obtained electrochemically as a film by cyclic voltammetry (CV) on platinum electrode. The analysis focuses essentially on the effect of two solvents acetonitrile and dichloromethane on the electrochemical behavior of the obtained polymer. The voltammograms show that the film of polyterthiophene can oxide and reduce in two solutions; in acetonitrile, the oxidation current intensity is more important than in dichloromethane. The impedance plots show the semicircle which is characteristic of charge-transfer resistance at the electrode/polymer interface at high frequency and the diffusion process at low frequency [13].
Grafted copolymer of polypyrrole has been synthesized by electrochemical polymerization of pyrrole in the presence of poly(para-chloromethylstyrene-costyrene- co-pyrrolemethylstyrene). The produced copolymer exhibits an electrical conductivity comparable to that of polypyrrole. This measurement showed that copolymer has excellent thermal stability. The response mechanism of this compound to sense a selection of gases and vapors was investigated, by measuring its electrical conductivity by four-point probe method. This gas sensor may have advantages over the other sensors in its ability to operate at room temperature, lower gas and vapous sensing concentration, suitable solubility, stability in air, sufficient diffusion, and selectivity [14].
This review highlights the recent progress made in the area of thermoelectric (TE) applications of conducting polymers and related composites. Several examples of such materials and their TE properties are discussed. TE properties of new poly(2,7-carbazole) derivatives are highlighted. References are also made to carbon nanotube/polymer composites and their improved electrical and TE performance. Studies on polymer/ inorganic materials composites have also taken a step forward and have shown very promising TE properties [15].
In this work, grafted polymer was modified with carbon nanotubes to fabrication grafted polymer electrode self modified with carbon nanotubes. The new grafted polymer electrode was electrochemically characterization in K3[Fe(CN)6] with KCl aqueous electrolyte by CV technique.
Experimental
Synthesis of Grafted Polymer modified with carbon nanotubes (GP/CNT)
Polystyrene was grafted with acrylonitrile as a monomer and modified with nano-deposit (carbon nanotubes) and ferrous ammonium sulfate (FAS) as a catalyst using gamma-irradiation. The new grafted polymer modified with carbon nanotubes has been investigated and characterized [3].
Instrument and Electroanalytical Methods
Electrochemical workstations of NuVant Systems Inc., USA (EZ stat series with potentiostat/glvanostat driven by electroanalytical measuring software) were connected to a PC computer in order to perform cyclic voltammetry (CV), chronoamperometry (CC), and chronoamperometry (CA). An Ag/AgCl (3 M NaCl) and platinum wire (1 mm diameter) were used as the reference and counter electrodes, respectively.
The working electrode used in this study was grafted polymer electrode self modified with carbon nanotubes (GPESMCNT). The voltammetric experiments were carried out with K3[Fe(CN)6] and KCl as supporting electrolyte. Solution was degassed with nitrogen gas for ten to fifteen minutes prior to recording the voltammogram.
Reagents
All reagents were analytical reagents or electrochemical grade purity. All solutions were prepared using distilled water. Unless otherwise specified, the supporting electrolyte was used 1M KCl in aqueous media at room temperature.
Fabrication the new grafted polymer electrode self modified with CNT (GPESMCNT)
GPESMCNT has been fabricated from grafted polymer modified with carbon nanotubes. The diameter of electrode was 3 cm. A hole was done (1mm) to allow 1cm length of platinum wire out from other side of electrode. A copper wire was then joined with the platinum wire. After that, all parts of fabricated electrode was covered with glassy tube and then fixed with epoxy resin.
Result and discussion
Electrochemical properties of grafted polymer electrode self modified with carbon nanotubes (GPESMCNT)
K3Fe(CN)6 solution is commonly used as a reference standard solution for the purpose of calibrating a voltammetric system in KCl aqueous solution. During the calibration process of an electroanalytical workstation (EZ stat) using glassy carbon electrode (GCE) and grafted polymer self modified with carbon nanotubes electrode (GPESMCNT) as working electrode. The current of Fe(II)/Fe(III) redox couple appears to be significantly enhanced by the GPESMCNT. The enhancement of oxidation-reduction current peaks +600μA and -200 μA, respectively is comparison of GCE at very weak redox current peaks of +70 μA and -60 μA respectively as show in figure 1a and 1b.
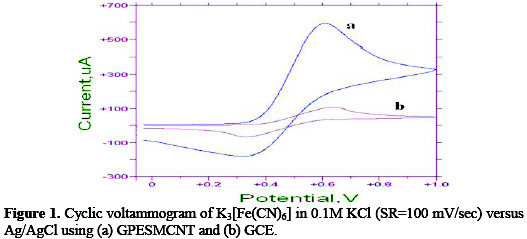
Effect of different Scan Rate
The effect of varying scan rates (SR) on the cyclic voltammograms using grafted polymer electrode self modified with CNT as working electrode in 1M KCl as a supporting electrolyte was studied with 1mM K3Fe(CN)6 over a scan rate ranging from 5 - 1000 mV/s. Oxidation and reduction currents of Fe(II)/Fe(III) couple increased with the scan rate due to heterogeneous kinetics and IR effect. Figure 2 is a reasonably linear dependence of GPESMCNT reduction current on the scan rate and is described by y=0.48x - 1.225, R2 =0.963.
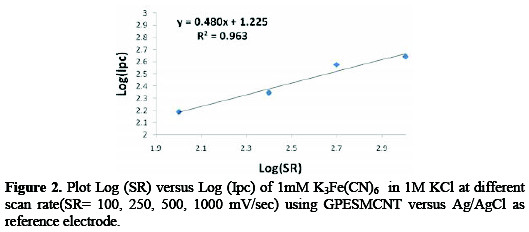
The slope of graph Log Ipc (reduction current) versus Log (SR) is 0.48; which is significantly differ from the theoretical value of half for diffusion-controlled process, indicating presence of a complex. The relationship between oxidative potential and scan rate of GPESMCNT, shows a reduction peak at 150mV in low scan rate but increased to 500mV at high scan rate (Linearly with Y=0.48X+150 (R2=0.963). Surface intercepts process at zero current produces zero current potential (E0,1) of 150 mV for the reduction of GPESMCNT.
Effect of Varying K3Fe(CN)6 Concentration
Figure 3 shows the linear current dependent on K3Fe(CN)6 concentration; observed at concentration range (5-10mM) which is described by the equation of y=18X+221.2 with R2=0.984.
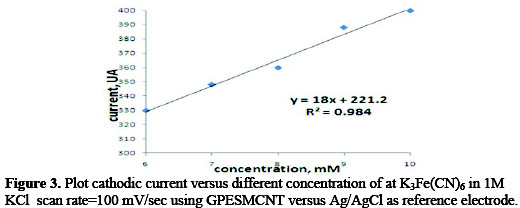
The slope of the linear line for K3Fe(CN)6 showed that a considerably high sensitivity response of18 μA/mM is readily obtained at GPESMCNT during cyclic voltammetry.
Reproducibility
The potential cycling of the redox of GPESMCNT in 1mM K3Fe(CN)6 and 1 M KCl aqueous solution as a supporting electrolyte was carried out during cyclic voltammetry. Continuous potential cycling did not seem to affect the redox current of GPESMCNT as the faradic activity appears reproducible even after 15 cycles, reflecting the stability and reproducibility at the surface of GPESMCNT.
Scanning Electron Microscopy (SEM) of GPE/CNT
Before electro-analysis, grafted polymer surface appears compact and nonporous. The uniformity of the grafted polymer surface slightly increases since occurrence of protrusion observed phase as shown in Figure 4a.
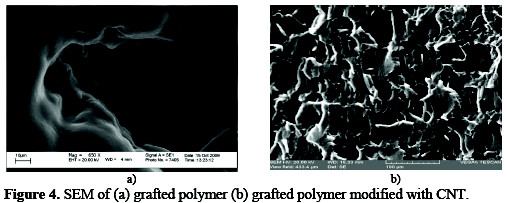
After modification with CNT, although many of the nano deposits with homogenous distribution of CNT still remain at about <1 μm as show in Figure 4b.
Atomic Force Microscopy (AFM)
The surface image of AFM in an area of 20 μm × 20 μm of the grafted polymer (polystyrene acrylonitrile) before and after modified with CNT as shown in Fig. 5.
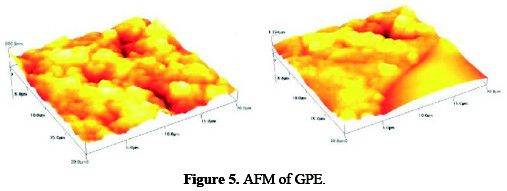
The surface of the electrode appeared to be compact and rough. According to AFM images, the average grain size and thickness of the film were estimated to be 11.23 μm and 28.69 μm, respectively.
Conclusions
A Grafted Polymer Electrode self modified with CNT (GPESMCNT) has an extended potential working region as a compared with solid electrodes and classical modification electrodes. The stability of GPESMCNT as a working electrode was evaluated by using K3Fe(CN)6 in KCl electrolyte. Redox peaks of Fe(II) / Fe(III) obtained at GPESMCNT showed high current as compared with bar GCE. Electro-catalytic activity of GPESMCNT is therefore evident in this study. GPESMCNT was studied by redox process of K3Fe(CN)6 in KCl solution during cyclic voltammetry. The redox peaks potential shifts slightly to less negative value by about 100 mV for oxidative peak and 50 mV for reductive peak with current enhancement of about 3-5 folds. The sensitivity under conditions of cyclic voltammetry is significantly dependent on the concentration and scan rate. Excellent reproducibility of the current is observed, provided a fabricated electrode is used for each experiment without cleaning.
References
1. Tan W T, Radhi M M, Ab Rahman M Z B, et al. J Appl Sci. 2010;10:139. [ Links ]
2. Radhi M M, Tan W T, Ab Rahman M Z et al. Sci Research Essays. 2012;7:790. [ Links ]
3. Radhi M M, Haider A J, Jameel Z N, et al. Research J Chem Sci. 2012;2:1. [ Links ]
4. Bhattacharya A, Misra B N. Prog Polym Sci. 2004;29:767. [ Links ]
5. Grande C D, Tria M C, Jiang G, et al. Reactive Funct Polym. 2011;71:938. [ Links ]
6. Cha S I, Kim K T, Lee K H, et al. Carbon. 2008;46:482. [ Links ]
7. Lee H J, Han S W, Kwon Y D, et al. Carbon. 2008;46:1850. [ Links ]
8. Lu W, Li N, Chen W, et al. Carbon. 2009;47:3337. [ Links ]
9. Wang L, Zhu D, Duan L, et al. Carbon. 2010;48:3906. [ Links ]
10. Natta G, Corradini P, Bassi I. Nuovo Cimento. 1960;15:68. [ Links ]
11. Ong T, Ng S, Chan H. Polymer. 2003;44:5597. [ Links ]
12. Tiwari D C, Jain R, Sahu G. Indian J Chem Techn. 2008;15:472. [ Links ]
13. Maouche N, Nessark B. Int J Electrochem. 2011;Article ID 670513. [ Links ]
14. Hoseini S H, Entezami A A. Iranian Polym J. 2005;14:101. [ Links ]
15. Dubey N, Leclerc M. J Polym Sci, Part b: Polym Phys. 2011;49:467. [ Links ]
*Corresponding author. E-mail address: mmradhi@yahoo.com
Received 04 April 2015; accepted 26 February 2016














