Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portugaliae Electrochimica Acta
Print version ISSN 0872-1904
Port. Electrochim. Acta vol.35 no.1 Coimbra Jan. 2017
https://doi.org/10.4152/pea.201701013
Corrosion Inhibition of Carbon Steel in Well Water by L-Cysteine-Zn2+ System
J. A. Thangakania,* , S. Rajendranb , J. Sathiabamac , R. J. Rathishd and S. Santhanaprabhad
a C.E.O.A. Mat. Hr. Sec. School, Madurai - 625 017, Tamil Nadu, India
b Department of Chemistry, RVS School of Engineering and Technology, Dindigul - 624 005 Tamil Nadu, India
c Corrosion Research Centre, Department of Chemistry, GTN Arts College, Dindigul - 624 005, Tamil Nadu, India
d PSNA College of Engineering and Technology, Dindigul, Tamil Nadu, India
Abstract
The environmental friendly inhibitor system L-cysteine-Zn2+ has been investigated by weight loss method. A synergistic effect exists between L-cysteine and Zn2+ system. The formulation consisting of 250 ppm of L-cysteine and 50 ppm of Zn2+ offers an excellent inhibition efficiency of 99%. Polarization study reveals that this formulation functions as anodic inhibitor. AC impedance spectra reveal that a protective film is formed on the metal surface. FTIR spectra study leads to the conclusion that the Fe2+L- cysteine complex formed on the anodic sites of the metal surface controlled the anodic reaction, and Zn(OH)2 formed on the cathodic sites of the metal surface controlled the cathodic reaction. A suitable mechanism of corrosion inhibition is proposed based on the results obtained from weight loss study and surface analysis technique. Synergism parameters have been calculated. They are found to be greater than 1, suggesting that a synergistic effect exists between L-cysteine and Zn2+.
Keywords: L-cysteine corrosion inhibitor, synergistic effect, carbon steel, well water, zinc ion, aminoacids.
Introduction
There has been a recent awareness about the health hazards of corrosion inhibition, and its best practices for health and safety. As hazardous chemicals have been restricted from contact with the environment, there has been an increasing look-out for non-toxic, eco-friendly corrosion inhibitors. Using inhibitors is one of the most pragmatic methods for protecting metals from corrosion.
Corrosion inhibitor is a chemical which, when added to the corrodible surface in optimum concentration, significantly decreases the corrosion rate of metals (or) alloys. However, many common corrosion inhibitors are highly toxic and hazardous to health, such as chromates [1], nitrite [2] and aromatic heterocyclic compounds [3]. Hence, environmentally safe inhibitors [4-6] are much recommended. Researchers have investigated and found that amino acids are both effective and environmental-friendly metal corrosion inhibitors, [6-13]. This is because aminoacids are non-toxic, biodegradable, economical and completely soluble in aqueous media, and produced with high purity at low cost. L-cysteine was selected as environmental-friendly corrosion inhibitor for the present project. The document presents some studies about the capability of amino acids to prevent corrosion in iron [14], steel [15-17], aluminium [18, 19], nickel [20] and copper [21-25]. Polarization and AC impedance spectra [26-30] and cyclic voltametry [19] have been studied by using amino acids. The adsorption of amino acids on carbon steel in an acidic environment has been researched by Adiyama et al. [31].
The aim of the present study is:
1. To assess the inhibition efficiency of L-cysteine in controlling the corrosion of carbon steel in the absence and presence of Zn2+.
2. To evaluate the protective film on carbon steel by FTIR spectrophotometry.
3. To study the mechanistic aspects by AC impedance and potentiodynamic polarization studies.
4. To propose a suitable mechanism for corrosion inhibition based on the results from the above study.
Experimental procedure
Preparation of specimens
Carbon steel specimens (0.0267% S, 0.067% P, 0.4% Mn, 0.1% C and iron), with the dimensions 1.0 cm × 4.0 cm × 0.2 cm, were polished to mirror finish, degreased with trichloroethylene, and used for weight loss method and surface examination studies.
Weight loss method
Carbon steel specimens, in triplicate, were immersed in 100 mL of well water and various concentrations of L-cysteine in the presence and absence of Zn2+ (as ZnSO4, 7H2O) for a period of seven days. The corrosion products were cleaned with Clarke`s solution [32]. The weight of the specimens before and after immersion was determined using a Shimadzu balance AY62. The corrosion inhibition efficiency was calculated with equation (1).
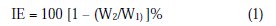
where W1 is the corrosion rate in the absence of the inhibitor, and W2 is the corrosion rate in the presence of an inhibitor.
Potentiodynamic polarization study
Potentiostatic polarization studies were carried out using a CHI electrochemical impedance analyzer, model 660 A. A three-electrode cell assembly was used. The working electrode was a rectangular specimen of carbon steel with one face of the electrode (1 cm2 area) exposed, and the rest shielded with red lacquer. A saturated calomel electrode (SCE) was used as reference electrode, and a rectangular platinum foil was used as counter electrode. Polarization curves were recorded using IR compensation. The results, such as Tafel slopes, and Icorr, Ecorr and LPR values were calculated. During the polarization study, the scan rate (v/s) was 0.01; hold time at Ef(s) was zero and quit time(s) was 2.
AC impedance measurements
A CHI electrochemical impedance analyzer (model 660A) was used for AC impedance measurements. A time interval of 5 to 10 minutes was given for the system to attain its open circuit potential. The real part Z' and imaginary part Z'' of the cell impedance were measured in ohms at various frequencies. Charge transfer resistance R1, double layer capacitance Cdl and impedance values were calculated.
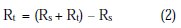
where Rs = solution resistance
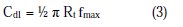
where fmax = maximum frequency
AC impedance spectra were recorded with initial E(v) = 0; high frequency (Hz) = 1; amplitude (v) = 0.05; and quiet time(s) = 2.
FTIR spectra
The structure of L-cysteine is shown in Fig. 1.

The carbon steel specimens immersed in various test solutions for one day were taken out and dried. The film formed on the metal surface was carefully removed and thoroughly mixed with KBr, so as to make it throughout uniform. The FTIR spectra were recorded in a Perkin-Elmer 1600 spectrophotometer.
Results and discussion
Analysis of results of the weight loss method
Inhibition efficiencies (IE%) of L-cysteine-Zn2+ systems in controlling corrosion of carbon steel immersed in well water, in the presence and absence of an inhibitor system (immersion period = 7 days), are given in Tables 1 to 3 and Schemes 1 to 3.
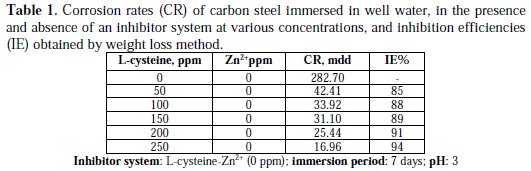
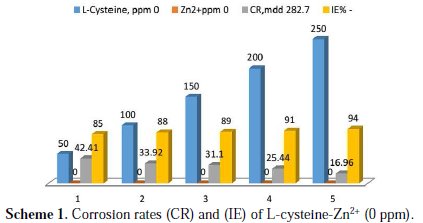
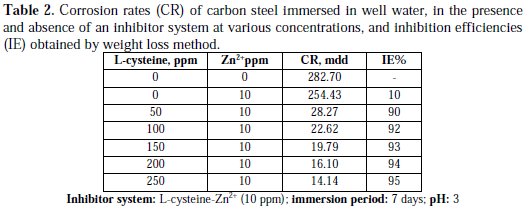
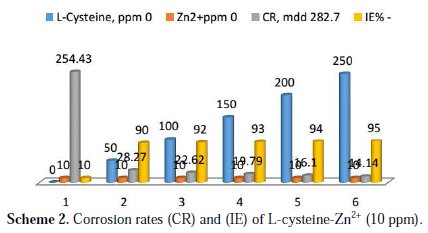
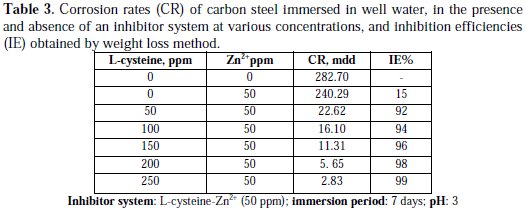
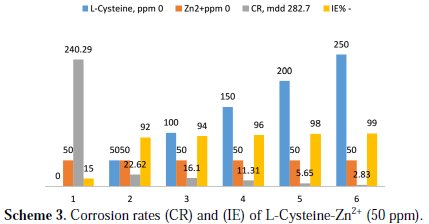
It is observed that L-cysteine alone has poor inhibition efficiency. In the presence of various concentrations of Zn2+ (10 and 50 ppm) the IE of L-cysteine increases. A synergistic effect exists between L-cysteine and Zn2+. For example, 50 ppm of L-cysteine has only 85% IE; 50 ppm of Zn2+ has 15% IE. However, their combination has 92% IE. This suggests a synergistic effect between L-cysteine and Zn2+ .
Synergism parameter (SI)
Synergism parameter (SI) has been used to know the synergistic effect between two inhibitors [33 -38]. Synergism parameter (SI) can be calculated as:
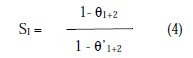
where θ = surface coverage, θ1+2 = (θ1+θ2)-(θ1 θ2), θ1 =surface coverage by L- cysteine, θ2 = surface coverage by Zn2+ , θ'1+2 = surface coverage by both L- cysteine and Zn2+, and where
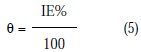
The synergism parameters of L-cysteine -Zn2+ system are given in Tables 4 and 5.
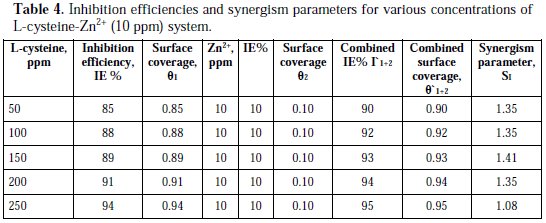
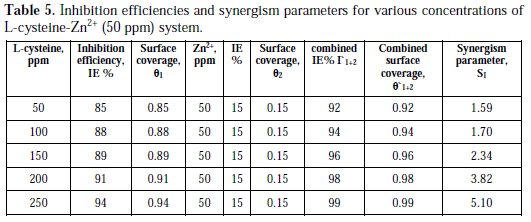
For different concentrations of inhibitors, SI approaches 1 when no interaction between the inhibitor and compounds exists. When SI > 1, it points to synergistic effects. If SI > 1, it is an indication that the synergistic effect is not significant. From Tables 4 and 5, it is observed that values of synergism parameters (SI) calculated from the surface coverage were found to be one and above. This indicates that a synergistic effect exists between L-cysteine and Zn2+ [35, 36, 38]. Thus, the enhancement of the inhibition efficiency caused by the addition of Zn2+ ions to L-cysteine is due to the synergistic effect.
Analysis of potentiodynamic polarization study (pH = 3)
Polarization study has been used to confirm the formation of a protective film formed on the metal surface during corrosion inhibition process [39-44]. If a protective film is formed on the metal surface, the linear polarization resistance value (LPR) increases, and corrosion current value (Icorr) decreases.
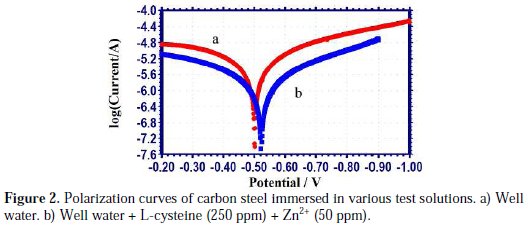
The potentiodynamic polarization curves of carbon steel immersed in well water, in the presence and absence of inhibitors, are shown in Fig. 2.
The corrosion parameters are given in Table 6.
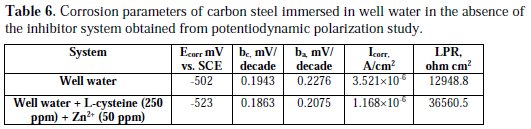
When carbon steel was immersed in well water, the corrosion potential was -502 mV vs. SCE. When L-cysteine (250 ppm) and Zn2+ (50 ppm) were added to the above system, the corrosion potential shifted to the cathodic side (-523 mV vs. SCE). This indicates that the inhibitor system predominantly controls the cathodic reaction (formation of OH-). In the presence of the inhibitor system, the corrosion potential shifted from -502 to -523 mV vs. SCE. This shift is with 50 mV/decade. Hence, it is concluded that the inhibitor system functions as a mixed type inhibitor system.
The anodic reaction (Fe → Fe2+ + 2e-) is controlled by the formation of Fe2+ inhibitor complex on the anodic sites of the metal surface.
The cathodic reaction (O2 + 2H2O + 4e- → 4OH-) is controlled by the formation of zinc hydroxide (Zn2+ + 2OH- → Zn(OH)2↓) on the cathodic sites of the metal surface. Thus, both the anodic reaction for the generation of Fe2+ , and the cathodic reaction for the formation of OH- are effectively controlled by the inhibitor system. This accounts for the mixed type of the inhibitor system and the synergistic effect between L-cysteine and Zn2+ system. Further, the LPR value increased from 12948.8 ohm cm2 to 36560.5 ohm cm2; and the corrosion current decreased from 3.521 × 10-6 A/cm2 to 1.168 × 10-6 A/cm2. Thus, the polarization study confirms the formation of a protective film on the metal surface.
Analysis of AC impedance spectra
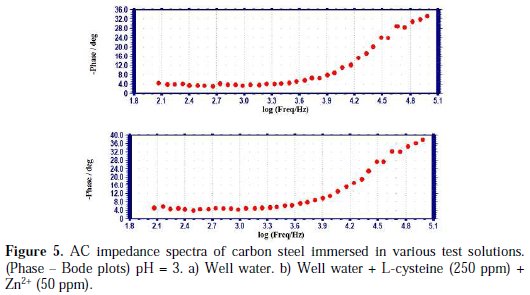
AC impedance spectra (electro chemical impedance spectra) have been used to confirm the formation of a protective film on the metal surface [45-47]. If a protective film is formed on the metal surface, charge transfer resistance (Rt) increases, double layer capacitance value (Cdl) decreases and impedance log (z/ohm) value increases. The phase angle also increases. The AC impedance spectra of carbon steel immersed in well water, in the presence and absence of inhibitors (L-cysteine-Zn2+), are shown in Figs. 3 (a, b) (Nyquist plots), Figs. 4 (a, b) and Figs. 5 (a, b) (Bode plots).
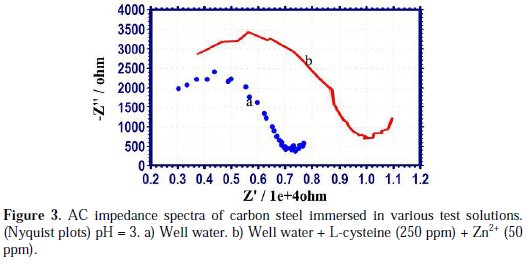
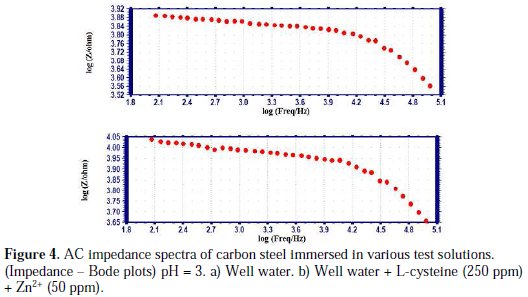
The AC impedance parameters, namely charge transfer resistance (Rt) and double layer capacitance (Cdl) derived from Nyquist plots are given in Table 7.
The impedance log (z/ohm) values derived from Bode plots are also given in Table 7.
It is observed that, when the inhibitors [L-cysteine (250 ppm) + Zn2+ (50 ppm)] are added, the charge transfer resistance (Rt) increases from 4737 ohm cm2 to 7336 ohm cm2. The Cdl value decreases from 1.056×10-9 F/cm2 to 0.6816×10-9 F/cm2. The impedance value [log (z/ohm)] increases from 3.8862 to 4.043. The phase angle increases from 34.00o to 39.00o. These results lead to the conclusion that a protective film is formed on the metal surface.
Analysis of FTIR spectra
FTIR spectra have been used to analyze the protective film formed on the metal surface [48-56]. The FTIR spectrum (KBr) of pure L-cysteine is shown in Fig. 6(a).
The -C=O stretching frequency of carboxyl group appears at 1640 cm-1. The -CN stretching frequency appears at 1131.7 cm-1. The -NH stretching frequency of the amine group appears at 2972.6 cm-1 [51-53]. The -CS stretching frequency appears at 634.8 cm-1.
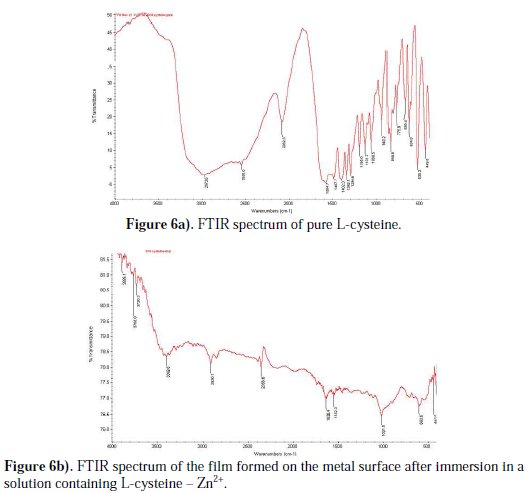
The FTIR spectrum of the film formed on the metal surface, after immersion in well water containing 250 ppm of L-cysteine and 50 ppm Zn2+, is shown in Fig. 6(b).
The -C=O stretching frequency has shifted from 1640 cm-1 to 1636.4 cm-1. The -CN stretching frequency has shifted from 1131.7 to 1021.0 cm-1 . The -NH stretching frequency has shifted from 2972.6 to 2920.1 cm-1. The -CS stretching frequency has shifted from 634.8 cm-1 to 603.6 cm-1. This observation suggests that L-cysteine has coordinated with Fe2+ , through the oxygen atom of the carboxyl group and nitrogen atom of the amine group, resulting in the formation of the Fe2+-L-cysteine complex on the anodic sites of the metal surface. The peak at 443.9 cm-1 corresponds to Zn-O stretching. The peak at 3399.8 cm-1 is due to OH stretching. This confirms that Zn(OH)2 is formed on the cathodic sites of the metal surface [45, 54-56]. Thus, the FTIR spectral study leads to the conclusion that the protective film consists of Fe2+-L-cysteine complex and Zn(OH)2. This accounts for the synergistic effect between the amino acid and Zn2+.
Mechanism of corrosion inhibition
The results of the weight-loss study show that the formulation consisting of 250 ppm L-Cys and 50 ppm of Zn2+ has 99% IE in controlling corrosion of carbon steel in well water. A synergistic effect exists between Zn2+ and L-Cys. Polarization study reveals that this formulation functions as a mixed type of inhibitor. AC impedance spectra reveal that a protective film is formed on the metal surface. FTIR spectra reveal that the protective film consists of Fe2+-L-Cys complex and Zn(OH)2. In order to explain these facts, the following mechanism of corrosion inhibition is proposed [57-68].
When the solution containing well water, 50 ppm Zn2+ and 250 ppm of L-Cys is prepared, there is formation of Zn2+-L-Cys complex in the solution. When carbon steel is immersed in this solution, the Zn2+-L-Cys complex diffuses from the bulk of the solution towards the metal surface.
Zn2+-L-Cys complex diffuses from the bulk solution to the surface of the metal and is converted into a Fe2+ -L-Cys complex, which is more stable than Zn2+-L- Cys [50].
On the metal surface Zn2+ -L-Cys complex is converted into Fe2+ -L-Cys on the anodic sites. Zn2+ is released.
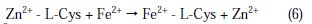
The released Zn2+ combines with OH- to form Zn(OH)2 on the cathodic sites [50].
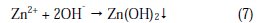
Thus, the protective film consists of the Fe2+-L-Cys complex and Zn(OH)2 [69,70].
Conclusions
1. The polarization study reveals that this formulation functions as a mixed type inhibitor system.
2. AC impedance spectra reveal that a protective film is formed on the metal surface.
3. The FTIR spectra study leads to the conclusion that the Fe2+-L-Cys complex formed on anodic sites of the metal surface controlled the anodic reaction, and that Zn(OH)2 formed on the cathodic sites of the metal surface controlled the cathodic reaction.
References
1. Baral A, Engelken RD. Environ Sci Policy. 2002;5:12. [ Links ]
2. Gaidis JM. Cement Concrete Comp. 2004;26:181. [ Links ]
3. Stupnisek-Lisac E, Bozic AL, Cafuk I. Corrosion. 1998;54:713. [ Links ]
4. El-Etre AY. Corrosion Sci. 2003;45:2485. [ Links ]
5. El-Etre AY. Corrosion Sci. 1998;45:1842. [ Links ]
6. Ashassi-Sorkhabi H, Majidi MR, Seyyedi K. Appl Surf Sci. 2004;225:176. [ Links ]
7. Ghasemi Z, Tizpar A. Appl Surf Sci. 2006;252:3667. [ Links ]
8. Zhang DQ, Gao LX, Zhou GD. J Appl Electrochem. 2005;35:1081. [ Links ]
9. Olivares O, Likhanova NV, Gomez BN, et al. Appl Surf Sci. 2006;252:2894. [ Links ]
10. Badawy WA, Ismail KM, Fathi AM. Electrochim Acta. 2006;51:4182. [ Links ]
11. Ismail KM. Electrochim Acta. 2007;52:7811. [ Links ]
12. Ashassi-Sorkhabi H, Ghasemi Z, Seifzadeh D. Appl Surf Sci. 2005;249:408. [ Links ]
13. Oguzie EE, Li Y, Wang FH. J Colloid Interf Sci. 2007;310:90. [ Links ]
14. Onal AA. Bull Electrochem. 1995;1:513. [ Links ]
15. Gomma G. Bull Electrochem. 1998;12:456. [ Links ]
16. Kalota D, Silverman D. Corrosion Sci. 1994;50:138. [ Links ]
17. Madkour L, Ghoneion M. Bull Electrochem. 1997;13:1. [ Links ]
18. Morad M, Hermas A, Aal M. J Chem Technol Biotechnol. 2002;77:486. [ Links ]
19. El-Shafei AA, Moussa MNH, El-Far AA. J Appl Electrochem. 1997;27:1075. [ Links ]
20. Fouda A, El-Semongy M. J Indian Chem Soc. 1982;59:89. [ Links ]
21. Aksut A, Bilgic S. Corros Sci. 1992;33:372. [ Links ]
22. Gomma G, Wahdan H. Mater Chem Phys. 1994;39:142. [ Links ]
23. Moretti G, Guidu F. Corrosion Sci. 2002;44:1995. [ Links ]
24. Rylkina M, Chikanova A, Trubacheva L, et al. Protect Metals. 1999;35:1987. [ Links ]
25. Baba H, Kodama T. Corrosion Sci. 1980;41:1995. [ Links ]
26. Ita BI. Bull Electrochem. 2005;21:319. [ Links ]
27. Canul MAP, Bartolo-Perez P. Surf Coat Technol. 2004;184:184. [ Links ]
28. Canul MAP, Echeverria M. Corrosion Eng Sci Technol. 2003;38:135. [ Links ]
29. Rajappa SK, Ventatesha TV. Turk J Chem. 2003;27:189. [ Links ]
30. Chi-Canul LP. Corrosion. 1999;55:948. [ Links ]
31. Akiyama A, Nobe N. J Electrochem Soc. 1970;117:999. [ Links ]
32. Wranglen G. An introduction to corrosion and protection of metals. New York: Chapman & Hall; 1985. [ Links ]
33. Gomma GK. Mater Chem Phys. 1998;55:241. [ Links ]
34. Aramaki K, Hackermann N. J. Electrochem. Soc. 1969;116:568. [ Links ]
35. Quraishi MA, Rawat J, Ajmal M. Corrosion. 1999;55:919. [ Links ]
36. Kanimozhi SA, Rejendran S. Int J Electrochem Soc. 2009;4:353. [ Links ]
37. Gopi D, Manimozhi S, Govindaraju KM, et al. J Appl Electrochem. 2007;37:439. [ Links ]
38. Rajendran S, Raj AJA, Joice MJ, et al. Corrosion Rev. 2004;22:233. [ Links ]
39. Roque JM, Padiyan T, Cruz J, et al. Corrosion Sci. 2008;50:614. [ Links ]
40. Benali O, Larabi L, Traisnel M, et al. Appl Surf Sci. 2007;253:6130. [ Links ]
41. Amar H, Braisaz T, Villemin D, et al. Mater Chem Phys. 2008;110:1. [ Links ]
42. Selvi JA, Rajendran S, Sri VG, et al. Port Electrochim Acta. 2009;27:1. [ Links ]
43. Rajendran S, Paulraj J, Rengan P. et al. J Dent Oral Hyg. 2009;1:1. [ Links ]
44. Kalman E, Felhosi I, Karman FH, et al. Corrosion and environmental degradation. In: Schutze M, editor. Weinheim: Wiky-VCh; 2000. [ Links ]
45. Zhang S, Tao Z, Li W, et al. Appl Surf Sci. 2009;255:6757. [ Links ]
46. Heakal FT, Fouda AS, Radwan MS. Mater Chem Phys. 2011;125:26. [ Links ]
47. Rajendran S, Devi MK, Regis APP, et al. Zastita Materijala. 2009;50:131. [ Links ]
48. Amar H, Braisaz T, Villemin D, et al. Mater Chem Phys. 2008;110:1. [ Links ]
49. Kalaivani R, Narayanasamy B, Selvi JA, et al. Port Electrochim Acta. 2009;27:177. [ Links ]
50. Sathiyabama J, Rajendran S, Selvi AA, et al. Open Corrosion J. 2009;2:76. [ Links ]
51. Silverstein RM, Bassler GC, Moril T. Spectrometric identification of organic compound. New York: John Wiley and Sons; 1981. [ Links ]
52. Cross AD. Introduction to practical infrared spectroscopy. London: Butterworths, Scientific Publication; 1990. [ Links ]
53. Nakamoto K. Infrared and Raman spectra of inorganic coordination compound. New York: Wiley Interscience; 1986. [ Links ]
54. Rajendran S, Apparao BV, Palaniswamy N. Bull Electrochem. 1996;12:15. [ Links ]
55. Sekine I, Hirakawa V. Corrosion. 1986;42. [ Links ]
56. Rajendran S, Apparao BV, Palaniswamy N. Proceedings 8th Eur Symp Corros Inhibitors. Ferrara; 1995. P.465. [ Links ]
57. Epshiba R, Regis APP, Rajendran S. Int J Nano Corrosion Sci Eng. 2014;1:1. [ Links ]
58. Kavitha N, Manjula P. Int J Nano Corrosion Sci Eng. 2014;1:31. [ Links ]
59. Nagalakshmi R, Nagarajan L, Rathish RJ, et al. Int J Nano Corrosion Sci Eng. 2014;1:39. [ Links ]
60. Thangakani JA, Rajendran S, Sathiabama J, et al. Int J Nano Corrosion Sci Eng. 2014;1:50. [ Links ]
61. Nithya A, Shanthy P, Vijaya N, et al. Int J Nano Corrosion Sci Eng. 2015;2:1. [ Links ]
62. Gowrani T, Manjula P, Baby N, et al. Int J Nano Corrosion Sci Eng. 2015;2:12. [ Links ]
63. Johar NK, Bhrara K, Epshiba R, et al. Int J Nano Corrosion Sci Eng. 2015;2:22. [ Links ]
64. Mary ACC, Rajendran S, Al-Hashem H, et al. Int J Nano Corrosion Sci Eng. 2015;2:42. [ Links ]
65. Thangakani JA, Rajendran S, Sathiabama J, et al. Int J Nano Corrosion Sci Eng. 2015;2:18. [ Links ]
66. Raja AS, Rajendran S, Sathiyabama J, et al. Int J Nano Corrosion Sci Eng. 2015;2:26. [ Links ]
67. Shanthy P, Rajendran S, Joany RM, et al. Int J Nano Corrosion Sci Eng. 2015;2:41. [ Links ]
68. Raja AS, Rajendran S, Sathiyabama J, et al. Int J Nano Corrosion Sci Eng. 2015;2:52. [ Links ]
69. Ruba HFG, Noreen AA, Sahayaraj JW, et al. Indian J Chem Technol. 2005;12:472. [ Links ]
70. Selvarani FR, Santhamadharasi S, Sahayaraj JW, et al. Indian J Chem Technol. 2005;12:472. [ Links ]
*Corresponding author. e-mail address: angelinthangakani@gmail.com
Received August 7, 2015; accepted October 30, 2016














