Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.35 no.6 Coimbra nov. 2017
https://doi.org/10.4152/pea.201706339
Corrosion Resistance of an SS 316L Alloy in Artificial Saliva in Presence of a Sparkle Fresh Toothpaste
Renita D'Souzaa,* , A. Chattreea and S. Rajendranb
a Department of Chemistry, SHIATS, Allahabad, 211007, UP, India
b Department of Chemistry, St Antony's College of Arts and Science for Women, Amala Annai Nagar, Thamaraipadi (Post), Dindigul - 624 005, India
Abstract
People are implanted with orthodontic wires made of different materials, to regulate their teeth. The various toothpastes that they use during the course of the treatment may have a corrosive effect on these materials. Hence, the main objective of this study was to evaluate the corrosion behaviour of an SS 316L alloy in artificial saliva in the presence of a sparkle fresh toothpaste. An electrochemical study has been used to investigate the corrosion behaviour of this alloy. Scanning electron microscopy (SEM) imaging gave the morphological data for the sample; however, by using X-ray spectroscopy in conjunction with SEM (EDAX), the elemental composition was determined. Further, the analysis of the protective film formed on the metal surface was done using UV-visible absorption and fluorescence spectra. The corrosion resistance of the SS 316L system in various solutions decreases in the following order: AS+ toothpaste> toothpaste>AS. For AS+ toothpaste system, LPR= 1813475 Ohm cm2; Icorr = 2.464 x 10-8 A/cm2; Rct =14961 Ohm cm2; Cdl= 3.4088 x10-10 F/cm2 and impedance = 4.397 log z/Ohm. The high corrosion resistance offered by the toothpaste is due to the formation of a protective film. It confirmed that the active principles of the toothpaste ingredients have co-ordinated with the SS 316L metal ions through their polar atoms to form a complex.
Keywords: Orthodontic appliances, toothpaste, dental alloys, impedance, resistance, ingredients.
Introduction
In dentistry, precious metals and alloys often used are Au, Ag, Pt and their alloys. They possess good cast ability, ductility and resistance to corrosion [1]. The escalating cost of precious metals throughout the 20th century has been one of the primary reasons for the development of base metal alloys for dental applications. In addition, these non-precious alloys were also found to provide better mechanical properties and aesthetics for some oral applications. Due to their superior mechanical properties and lower density, certain base metal alloy systems are preferred. Stainless steels, nickel-chromium, cobalt-chromium, titanium, and nickel-titanium alloys are some of the base metal alloy systems most commonly used in dentistry of today [2].
Stainless steel alloys
Stainless steel has been successfully used in dentistry for almost a century. The first stainless steel used for implants contained ∼18wt% Cr and ∼8wt% Ni, which made it stronger than steel and more resistant to corrosion. Further addition of molybdenum (Mo) has improved stainless steels (known as type 316). Afterwards, the carbon (C) content has been reduced from 0.08 to 0.03 wt%, which improved stainless steel (named as 316L) corrosion resistance to a chloride solution [1, 3].
Physical properties of metals
One important criterion in metals selection is the consideration of their physical properties, such as density, melting point, specific heat, thermal conductivity, thermal expansion and corrosion. For many applications, one of the most important considerations is their deterioration by corrosion. Corrosion of metal depends on the metals composition and the corrosive media in the surrounding environment [3].
Chromium has a very strong affinity with oxygen, resulting in the creation of chromium oxide on the surface of stainless steel, when it is exposed to oxygen. Chromium oxide is a very thin layer that prevents further oxidation of stainless steel. Even if stainless steel is scratched and the chromium oxide layer is removed, a new chromium oxide layer will form and protect the remaining stainless steel beneath it. The chromium oxide layer will continue to protect stainless steel and prevent it from undergoing corrosion, as long as there is sufficient chromium present in it [4].
There are several metals and metal alloys used in dentistry and orthodontic applications. Corrosion of orthodontic appliances has been thoroughly studied [5-10]. The oral cavity represents a harsh environment for orthodontic appliances of any kind [11]. The traces of corrosion on the surfaces of metals used in any application can be formed after a period of time depending on the mouth environment [12]. The corrosion process occurs as a result either of the loss of metal ions directly into the solution or of the progressive dissolution of the surface films, as a rule oxide or sulphide [13]. Corrosion resistance is one of the important features of dental materials, because after introducing the metallic biomaterials into the human body, they are subject to a corrosive medium [14]. Orthodontic wires are recommended by the dentists to regulate the arrangement of teeth. People with these orthodontic wires have to daily brush their teeth. The toothpaste that they use may corrode the orthodontic wires in the oral environment. Hence, there is a need to investigate the influence of various toothpastes on the corrosion resistance of orthodontic wires made of many metals and alloys. The following work was undertaken to study the corrosion behaviour of an SS 316L alloy in artificial saliva, in the absence and presence of a sparkle fresh toothpaste.
Experimental
Materials
The metal specimens, namely, SS 316L, were chosen for the present study. The study was carried out in the presence of artificial saliva (AS) using a sparkle fresh toothpaste. The composition of SS 316L is Cr (18%), Ni (12%), Mo (2.5%), C (<0.03%) and balance is Fe [15]. The composition of artificial saliva in g/L-1 is KCl (0.4), NaCl (0.4), CaCl2.2H2O (0.906), NaH2PO4.2H2O (0.690), Na2S.9H2O (0.005) and urea (1.0) [16-18].
The ingredients of the sparkle fresh toothpaste are sodium monofluorophosphate (active ingredients), calcium carbonate, carboxymethyl cellulose, glycerine, hydrated silica, sodium benzoate, sodium lauryl sulfate, sodium saccharin, sorbitol, tetra sodium pyrophosphate and water (inactive ingredients).
Methods
Potentiodynamic polarization
Polarization studies were carried out in a CHI-electrochemical workstation with impedance, model 660A. A three-electrode cell assembly was used. The working electrode used was a thin wire metal specimen. A saturated calomel electrode (SCE) was the reference electrode, and platinum was the counter electrode. IR contribution was minimized by placing the reference electrode close to the working electrode. To attain a steady state open circuit potential, a time interval of about 5 minutes was given for the working electrode. The corrosion parameters such as corrosion potential (Ecorr), corrosion current (Icorr), Tafel slopes (anodic = ba and cathodic = bc) and linear polarization resistance (LPR) were calculated. During the polarization study, the scan rate (V/s) was 0.005; hold time at Ef (s) was zero and quite time (s) was 2.
AC impedance measurements
The measure of the ability of a circuit to resist the flow of electrical current is known as impedance. By applying an AC potential to an electrochemical cell and then measuring the current through the cell, the electrochemical impedance is usually measured using a small excitation signal. The instrument used for the polarization study was also used to record AC impedance spectra. The cell setup was also the same. The real part (Z') and imaginary part (Z'') of the cell impedance were measured in Ohms at various frequencies. Values of the charge transfer resistance (Rt) and double layer capacitance (Cdl) were calculated from the Nyquist plot and the impedance; log (z/Ohm) value was calculated from Bode plots. During AC, impedance spectra were recorded: the scan rate (V/s) was 0.005; hold time at Ef(s) was zero and quite time (s) was 2. The value of charge transfer resistance (Rt) and double layer capacitance (Cdl) were calculated from Nyquist plot.
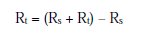
(where Rs = solution resistance, Rt =charge transfer resistance)
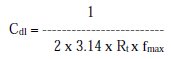
where fmax= frequency at maximum imaginary impedance.
UV-visible absorption spectra of solutions
The possibility of the formation of a metal - inhibitor complex in a solution was examined by recording its UV-visible absorption spectra for the blank, the inhibitor and the best system solution using an Analytic Jena Specord S-100, UV -visible spectrometer.
Fluorescence spectroscopy
Fluorescence spectra of solutions, blank, the inhibitor and the best system were recorded by using a Jasco-6300 spectrofluorometer.
Scanning Electron Microscopic studies (SEM)
The surface morphology measurements of the thin wire metal specimen were examined using Tescon, Vega3, and a USA computer controlled scanning electron microscope. The surface morphology was examined for the thin wire metal specimen in absence and in presence of the inhibitor system. The specimen immersed in the best system for a period of one day was removed, rinsed with double distilled water, dried and observed in a scanning electron microscope to examine the surface morphology.
Energy Dispersive Analysis of X-rays (EDAX)
SEM imaging gives the morphological data for a sample; however, by using X- ray spectroscopy in conjunction with SEM, the elemental composition can be determined. The elements present in a material are determined by an EDAX spectrum. An energy dispersive X-ray analyzer (EDAX) [Brucker, Nano, GMBH, Germany] unit attached to the SEM machine was used to carry out the elemental analysis of the metal surface.
Results and discussion
Analysis of potentiodynamic polarization study
The polarization curves of SS 316L immersed in various test solutions are shown in Fig. 1.
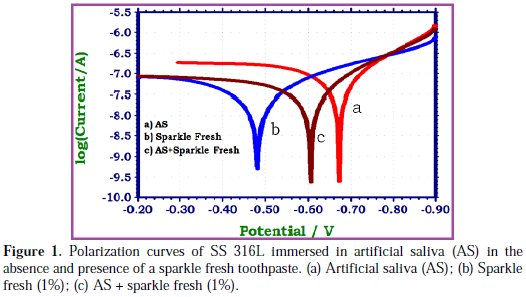
The corrosion parameters, namely, corrosion potential (Ecorr), Tafel slopes (bc = cathodic; ba = anodic), linear polarization resistance (LPR) and corrosion current (Icorr) derived from polarization curves are given in Table 1.

This observes that, when SS 316L is immersed in AS, the corrosion potential is -672 mV vs. SCE. The linear polarization resistance value is 630154 Ohm cm2. The corrosion current is 6.872 × 10-8 A/cm2. When SS 316L is immersed in an aqueous solution of (1%) sparkle fresh toothpaste, the corrosion potential is shifted to the noble side (-606 mV vs. SCE). The linear polarization resistance (LPR) value increases from 630154 Ohm cm2 to 1347241 Ohm cm2. The corrosion current decreases from 6.872 × 10-8 A/cm2 to 3.272 x 10-8 A/cm2. These observations indicate that the anodic reaction is predominantly controlled. A protective film is formed on the metal surface. Hence, the linear polarization resistance (LPR) value increases and corrosion current (Icorr) decreases. The protective film may probably consist of complexes formed between stainless steel ions and the active principles of the toothpaste ingredients. When SS 316L is immersed in an aqueous solution consisting of AS and the 1% paste solutions, the corrosion potential is shifted to the anodic side (-482 mV vs. SCE). Hence, it is inferred that the anodic reactions are predominantly controlled. Further, the linear polarization resistance (LPR) value increases from 630154 Ohm cm2 to 1813475 Ohm cm2. Corrosion current decreases from 6.872 × 10-8 A/cm2 to 2.464 × 10-8A/cm2. These observations indicate that the corrosion resistance of SS 316L increases when it is immersed in AS containing aqueous solutions of the sparkle fresh toothpaste.
Analysis of AC impedance spectra
The AC impedance spectra of SS 316L immersed in various test solutions are shown in Figs. 2, 3, 4 and 5.
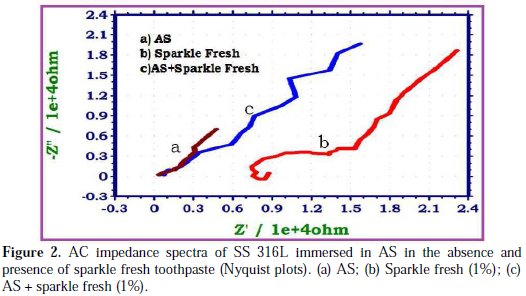
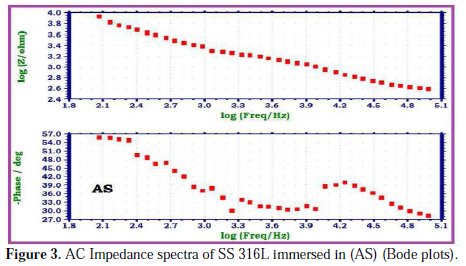
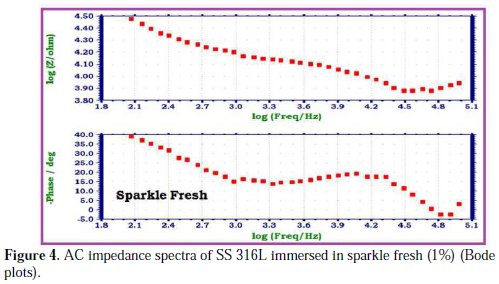
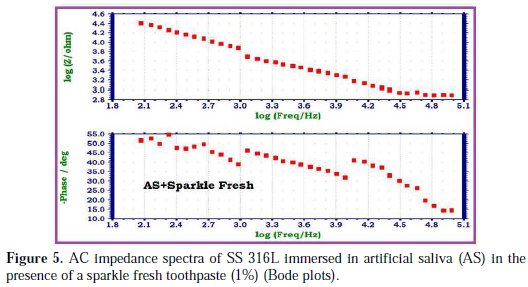
The Nyquist plots are shown in Fig. 2. The Bode plots are shown in Figs. 3, 4 and 5. The corrosion parameters derived from these plots are shown in Table 2.
When SS 316L is immersed in AS, the charge transfer resistance (Rt) value is 4397 Ohm cm2, the double layer capacitance (Cdl) value is 11.598 x10-10 F/cm2 and the impedance (log z/Ohm) value is 3.928. When SS 316L is immersed in aqueous solutions of (1%) sparkle fresh toothpaste, the charge transfer resistance (Rt) value increases from 4397 Ohm cm2 to 14380 Ohm cm2; the double layer capacitance value (Cdl) decreases from 11.598 × 10-10 F/cm 2 to 3.5465 × 10-10 F/cm2, and the impedance value (log z/Ohm) increases from 3.928 to 4.473. These observations indicate that a protective film is formed on the metal surface when SS 316L is immersed in aqueous solutions of a sparkle fresh toothpaste. The protective film prevents the transfer of electrons from the metal surface to the bulk of the solutions. Hence, corrosion resistance increases and the rate of corrosion decreases. The protective film probably consists of stainless steel ions, and the active principle of the toothpaste's ingredients.
When SS 316L is immersed in AS containing an aqueous solution of sparkle fresh toothpaste, the charge transfer resistance (Rt) value increases from 4397 Ohm cm2 to 14961 Ohm cm2; the double layer capacitance (Cdl) value decreases from 11.598 × 10-10 F/cm2 to 3.4088 × 10-10 F/cm2; and the impedance (log z/Ohm) value increases from 3.928 to 4.397. It is inferred that, in presence of AS containing sparkle fresh toothpaste, the corrosion resistance of SS 316L increases.
Analysis of UV-visible absorption spectra
The UV-visible absorption spectrum is used to confirm the protective film formed on the metal surface. The UV-visible absorption spectrum of artificial saliva is shown in Fig. 6(a).
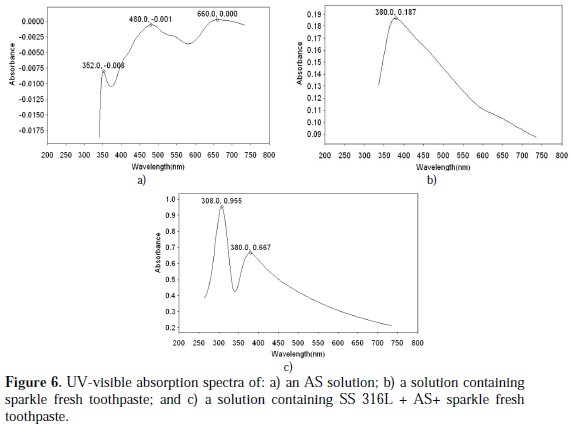
Peaks appear at 352 nm, 480 nm and 660 nm. The UV-visible absorption spectrum of the toothpaste solution is shown in Fig. 6(b). A peak appears at 380 nm. The UV-visible absorption spectrum of the solution of AS toothpaste system, where in SS 316L has been immersed for one day, is shown in Fig. 6(c).
A peak appears at 380 nm. There is no shift in the position of λmax of the toothpaste system. This indicates that the SS 316L alloy has not undergone corrosion in presence of the saliva and toothpaste system. Had there been corrosion, there would have been a shift in the position of λmax. The change in intensity at 380 nm may be attributed to the fact that there is no electronic transition because of the co-ordination of the active principles of the toothpaste's ingredients with the SS 316L alloy.
Analysis of fluorescence spectra
Fluorescence spectra are used to detect the presence of the metal-inhibitor complex formed on the surface of the SS 316L alloy. The fluorescence spectrum (λex = 300 nm) of AS is shown in Fig. 7(a).
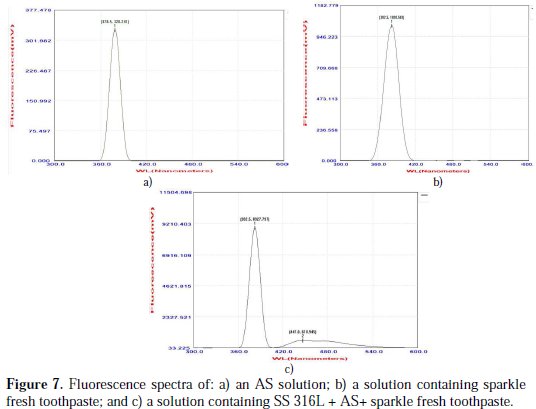
A peak appears at 378.5 nm. The fluorescence spectrum (λex = 300 nm) of an aqueous solution of sparkle fresh toothpaste is shown in Fig. 7(b). Emission takes place at 381.5 nm. SS 316L was immersed in an aqueous solution containing AS and the sparkle fresh toothpaste. A solution was obtained. The fluorescence spectrum (λex = 300 nm) of this solution is shown in Fig. 7(c).
The peaks appear at 382.5 nm and 447.0 nm. The shift in λmax is not substantial. This indicates that SS 18/8 has not undergone substantial corrosion in presence of AS and the toothpaste. The slight shift in the λmax value may be due to the release of some Cu ions in this system. This indicates that a protective film is formed on the metal surface. There is UV-blue emission.
Analysis of Scanning Electron Microscopy (SEM)
SEM images for SS 316L alloy in absence and presence of the system are shown in Fig. 8(a) and 8(b).
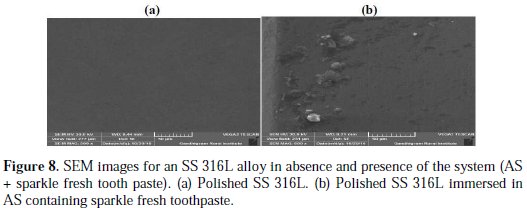
The surface is found to be smooth only for pure polished metals; the system surface has become rough due to the presence of a film deposited on the metal surface. This protective film is due to the deposition of the active principles of the ingredients present in the toothpaste.
The EDAX spectra are shown in Fig. 9(a) and 9(b).
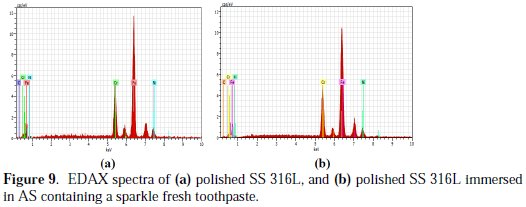
It is seen from the EDAX spectra that Fe, Cr, Ni and C are present in both absence and presence of the inhibitor (Table 3 and 4).
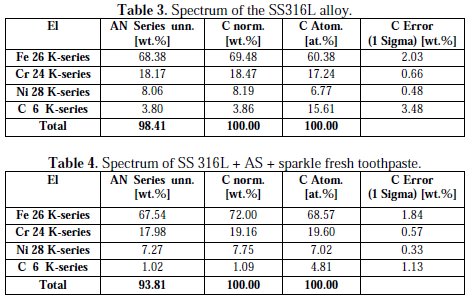
But the weight percentage of these elements has changed after immersion in the AS containing a sparkle fresh toothpaste. The weight percentage of Fe and Cr has increased as weight percentage of C decreases. The intensity of the peak of Fe is reduced as the active principles of the toothpaste's ingredients form a protective film on the metal surface, thus preventing the corrosion of the SS 316L alloy.
Conclusions
In presence of a sparkle fresh toothpaste, corrosion resistance of the SS 316L alloy increases. Hence, it is recommended that people implanted with orthodontic wires made of SS 316L alloy use sparkle fresh toothpaste to clean their teeth without any hesitation. So, dentists can recommend this toothpaste to their patients.
References
1. Dongre, Choudaha S. Int J Innov Eng Res. 2016;6:1. [ Links ]
2. Roach M. Dent Clin North Am. 2007;51:603. [ Links ]
3. Hermawan H, Ramdan D, Djuansjah JRP. Intech. 2011;17:411. [ Links ]
4. Wensley A. Mater Sci Res Innov. 2015. [ Links ]
5. Walker MP, White RJ, Kula KS. Am J Orthod Dentof Orthop. 2005;127:662. [ Links ]
6. Kaneko K, Yokoyama K, Moriyama K, et al. Angle Orthodontist. 2004;74:487. [ Links ]
7. Yokoyama K, Kaneko K, Ogawa T, et al. Biomaterials. 2004;26:101. [ Links ]
8. Schiff N, Grosgogeat B, Lissac M, et al. Biomaterials. 2004;25:4535. [ Links ]
9. Ogawa T, Yokoyama K, Asaoka K, et al. Biomaterials. 2004;25:2419. [ Links ]
10. Kaneko K, Yokoyama K, Moriyama K, et al. Biomaterials. 2003;24:2113. [ Links ]
11. McCann HC. Inorganic components of salivary secretions in art and science of dental caries research. Harris RS, editor. New York: Academic Press; 1968. p.55-70.
12. House K, Sernetz F, Dymock D, et al. Am J Orthodontics Dentofacial Orthopedics. 2008;133:584. [ Links ]
13. Unal, Zor S, Atapek H. Mater Sci. 2012;47:830. [ Links ]
14. Hancu V, Comaneanu RM, Coman C, et al. Rev Chim (Bucharest). 2014;65:706. [ Links ]
15. Rajendran S, Uma V, Krishnaveni A, et al. Arab J Sci Eng. 2009;34:147. [ Links ]
16. S. Rajendran S, Paulraj J, Rengan P, et al. J Dent Oral Hug. 2009;1:1.
17. Meyer M. Corros Sci. 1977;17:971. [ Links ]
18. Brett MAC, Muresan I. Eng Mater. 2002;230-232:459. [ Links ]
Acknowledgements
The authors are thankful to their respective departments for the help and encouragement to carry out this research.
*Corresponding author. E-mail address: renitavinaya@yahoo.com
Received November 02, 2016; accepted April 15, 2017














