Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portugaliae Electrochimica Acta
Print version ISSN 0872-1904
Port. Electrochim. Acta vol.38 no.1 Coimbra Jan. 2020
https://doi.org/10.4152/pea.202001019
ARTIGOS
Thermometric and Gravimetric Analyses of Aluminum Corrosion Control in a HCl Medium, Using Ricinus Communis Extract
O.D. Onukwulia, M. Omotiomab* and I. Obiora-Okafob
a Department of Chemical Engineering, Nnamdi Azikiwe University, Awka, Nigeria
b Department of Chemical Engineering, Madonna University, Akpugo Campus, Nigeria
ABSTRACT
This work presents the thermometric and gravimetric analyses of aluminum corrosion control in a HCl medium, using Ricinus communis (castor oil) extract. The obtained plant extract was subjected to phytochemical analyses for the identification of various sample constituents. Applying the thermometric technique, reaction values for the aluminum dissolution in free and inhibited HCl media were used to determine the extract inhibition efficiency. The gravimetric method was carried out using one factor at a time, and response surface methodology. Thermodynamic parameters of activation energy, heat of adsorption and free Gibbs energy of the corrosion inhibition process were determined. Central composite design of Design Expert Software was employed in the response surface methodology. The results analysis revealed that Ricinus communis extract contained phytochemicals of alkaloids, cardiac glycosides, flavonoids, phenolics, phytates, saponins and tanins. The extract adsorption onto the aluminum surface obeyed the physical adsorption mechanism; the activation energy was lower than the threshold value of 80 kJ/mol required for chemisorption. A quadratic model adequately described the relationship between the inhibition efficiency and the inhibitor concentration, temperature and time factors. The thermometric and gravimetric techniques recorded high inhibition efficiencies of 83.93% and 82.61%, respectively, showing that the Ricinus communis extract can be employed to control aluminum corrosion in an HCl medium.
Keywords: aluminum, corrosion control, HCl and Ricinus communis.
Introduction
Plants’ phytochemicals are of great importance in the corrosion control of metals in acidic media [1]. Phytochemicals can be correlated to the plants’ antioxidant capacity [2-3]. Aluminum corrosion control is needed, because of its high demand in engineering applications. It is used for the construction of reaction vessels, pipes, machinery and chemical batteries, due to its advantages of low cost, lightness and good corrosion resistance at moderate temperatures [4-5]. The formation of a passive oxide film on the aluminum surface is responsible for its resistance to corrosion, but the surface film is amphoteric, and it substantially dissolves when the metal is exposed to high concentrations of acids or basses [6-8]. Previous studies on aluminum corrosion control in corrosive media, using plant-based inhibitors, include the study of tobacco and kola tree extracts effects on the corrosion inhibition of aluminum alloy in sulphuric acid [9]. Gravimetric and metallographic methods were employed in this study. It was observed that the corrosion inhibition was associated with the protective film formed on the aluminum alloy’s surface by the complex chemical constituents of the extracts. Also, the inhibition effect of Dendrocalamus brandisii leaves extract on aluminium in HCl and H3PO4 solutions has been reported [8]. The extract adsorption onto the aluminum surface obeyed Langmuir isotherm in both acids. The Dendrocalamus brandisii leaves extract acted as a cathodic inhibitor in HCl. From the review of previous works, there was a need to screen the plant extracts’ phytochemicals, and model the extract inhibition efficiency. The aim of this work was to study aluminum corrosion control in a HCl medium, using Ricinus communis as inhibitor. It involved phytochemical screening and the determination of the Ricinus communis extract inhibition efficiency.
Materials and methods
Analytical grade 1.0 M HCl was used in this study. Leaves of Ricinus communis (castor oil plant) were obtained from Akpugo, Enugu State, Nigeria. The plant leaves were sun-dried for three days. The dried leaves were ground to increase the surface area, and stored in closed containers. 30 g of the ground leaves (particle size of 0.85 mm) were measured and soaked in 1000 mL of ethanol (99.7% v/v), for 48 hours. Afterwards, the mixture was filtered. The leaves’ extract was obtained by evaporating the ethanol from the filtrate. The extract was used for the corrosion inhibition study. The Al coupons with a chemical composition of Si (0.25%), Fe (0.02%), Zn (0.05%), Mn (0.04%), Mg (0.03%), V (0.04%), Ti (0.02%), Cu (0.03%), Cr (0.02%) and Al (99.5%) were cleaned, and then polished with emery paper. To remove any oil and organic impurities, the coupons were degreased with acetone, washed with distilled water, dried in air, and then stored in desiccators.
Phytochemical analysis of the Ricinus communisextract
Methods used by previous authors [10-11] were adopted for the qualitative and quantitative analysis of the plant leaves’ phytochemicals. The obtained plant extract was subject to a phytochemical test for the identification of various sample constituents. For the alkaloids detection, a small portion of solvent free cashew extract was transferred into the test tube. A few drops of dilute hydrochloric acid were added, stirred and then filtered. The filtrate was carefully tested with alkaloid reagents: Mayer’s reagent (Cream ppt) and Dragendorff’s reagent (Orange-brown ppt). For the detection of cardiac glycosides, 1 mL of the extract, 5 mL of water and 2 mL of glacial acetic acid were mixed in a vessel. One drop of FeCl3 was added. Then, 1 mL of concentrated H2SO4 was added, after which a brown ring appeared. For the determination of flavonoids, 25 mL of water were added to 1 g of the sample, which was put in an oven at 100 ºC, for 15 min. 5 mL of NH4OH were added to 2 mL of the extract. Then, 1 mL of concentrated H2SO4 was added, and the solution turned yellow.
The phenolics presence was determined by adding a few drops of 1% (w/v) ferric chloride solution, followed by 1% (w/v) of gelatin in sodium chloride, with the same concentration. The formation of a precipitate indicated the phenolics presence. For the saponins determination, 1 g of the sample was boiled in 40 mL of water, and filtered. 10 mL of the filtrate were vigorously shaken. The formation of froth was noticed. 3 drops of oil were added, and the mixture was shaken. The oil emulsion was noticed. For the determination of tannins, 1 g of the sample was added to 25 mL of water. It was then put in an oven at 100 ºC, for 15 min. To 1 mL of the extract, 10 mL of water were added, and then boiled. A few drops of 0.1% FeCl3 were added, and then the green color appeared.
Thermometric method of study
In the thermometric method of study the technique used by previous researchers was adopted [12-14]. The aluminum samples were immersed in beakers containing inhibited and uninhibited HCl media. The beakers were placed in a thermostat set at 30 ºC. The progress of the corrosion reaction was monitored, and the temperatures of the system containing the aluminum sample and the test solution were regularly recorded, until a steady temperature value was obtained. Equations (1) and (2) were used for the determination of the reaction and inhibition efficiency values.

where Tm and Ti are the maximum and initial temperatures (in ºC), respectively, and t is the time in elapsed minutes, to reach Tm.
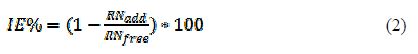
where RNfree and RNadd are the reaction numbers for Al dissolution in free and inhibited HCl media, respectively.
Gravimetric method of study
The gravimetric method of study was carried out using one factor at a time, and response surface methodology. Thermodynamic parameters of activation energy, heat of adsorption and free Gibbs energy of the corrosion inhibition process were determined. Central composite design of Design Expert Software was employed in the response surface methodology. The weight loss (?w), corrosion rate (CR), inhibition efficiency (IE) and degree of surface coverage were calculated using standard equations (3), (4), (5) and (6), respectively.
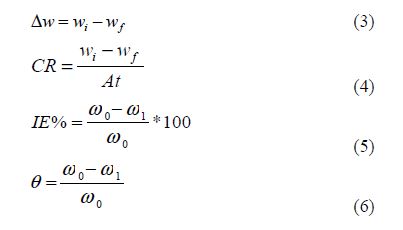
where wi and wf are the initial and final weight of Al samples, respectively, and w1 and w0 are the weight loss values in the inhibitor presence and absence, respectively. A is the total area of the Al sample and t is the immersion time.
The activation energy and heat of adsorption for Al corrosion inhibition in HCl with Ricinus communis extract are presented in table 5. A linearized Arrhenius model of Equation (7) was used to determine the activation energy (Ea) (kJmol-1), while Equation (8) was employed to evaluate the heat of adsorption (Qads) (kJmol-1) [15, 16, 17 and 18].
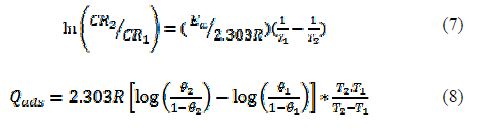
where Al corrosion rates at T1 and T2 are CR1 and CR2, R is the gas constant, and θ1 and θ2 are the degree of surface coverage at temperatures T1 and T2, respectively.
The data obtained for the degree of surface coverage were used to test the applicability of Langmuir, Frumkin, Temkin and Flory-Huggins adsorption isotherms expressed in Equations (9), (10), (11) and (12), respectively [8, 14, 17, 19-20]. The free energy of adsorption (?Gads) was calculated using Equation (13).
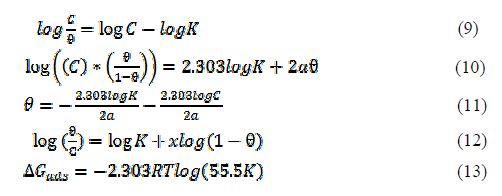
where K is the adsorption equilibrium constant, C is the inhibitor concentration, θ is the degree of surface coverage, α is the lateral interaction term describing the interaction in an adsorbed layer, a is the attractive parameter, R is the universal gas constant and T is the temperature.
Results and discussion
Qualitative and quantitative results of the extract’s phytochemicals
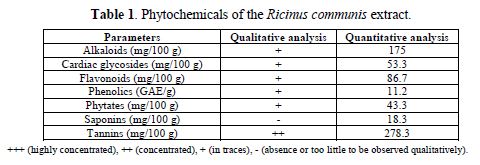
In table 1, the qualitative and quantitative analysis of the Ricinus communis extract showed that the phytochemicals of alkaloids, cardiac glycosides, flavonoids, phenolics, phytates, saponins and tanins were present in the extract, at various degrees. The qualitative results of the extract’s phytochemicals are denoted with the following symbols: +++ (highly concentrated), ++ (concentrated), + (in traces), and – (absence or too low to be qualitatively observed). The results of the quantitative analysis are in agreement with the trends observed in the qualitative analysis. The phytochemicals presence signifies that the Ricinus communis extract is a potential corrosion inhibitor. This is in agreement with the assertion that phytochemicals can be correlated to the plant’s antioxidant capacity [2-3].
Results of the thermometric method
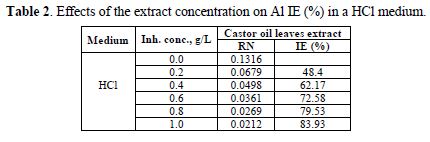
The results of the thermodynamic measurements are presented in table 2. A higher concentration increases the inhibition efficiency of the Ricinus communis extract. Highest inhibition efficiency of 83.93% was obtained at 1.0 g/L. It is an indication that Ricinus communis extract is a suitable inhibitor of Al corrosion inhibition in an HCl medium.
Results of the gravimetric method
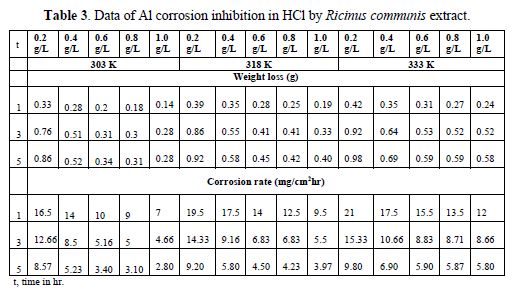
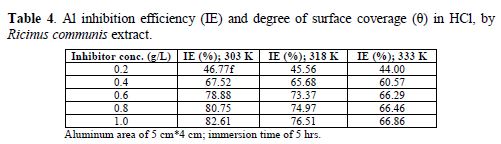
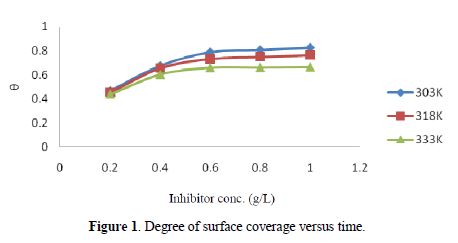
The results of the gravimetric method are shown in table 3. Al weight loss and corrosion rate reduced with higher inhibitor concentrations, but increased with higher temperatures. In table 4, the inhibition efficiency increased with higher extract concentrations, but decreased with an increase in temperatures. The inhibition efficiency of 82.61 % was obtained at the inhibitor concentration of 1.0 g/L, temperature of 303 K and time of 5 hrs. Also, the degree of surface coverage increased with higher inhibitor concentrations, but decreased with an increase in temperatures (Fig. 1).
Adsorption and thermodynamic properties
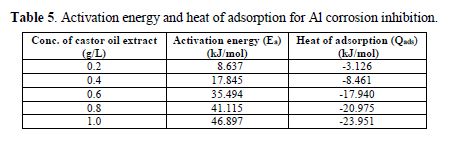
The activation energy and heat of adsorption for Al corrosion inhibition in HCl by Ricinus communis extract are shown in table 5. The activation energy increased with higher concentrations. The activation energy is lower than the threshold value of 80 kJ/mol required for chemisorption. The negative sign of the heat of adsorption showed that the inhibitive process was an exothermic reaction.
Adsorption parameters for aluminum corrosion inhibition in HCl by Ricinus communis leaves extract are shown in table 6.
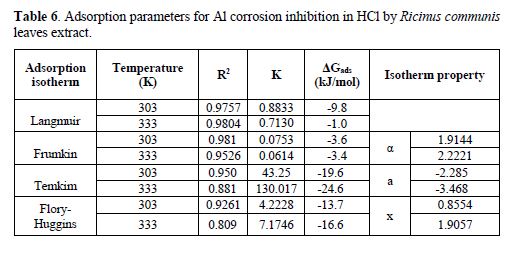
The free energy of adsorption (?Gads) values were lower than the threshold value of -40 kJ/mol. The R2 values are close to 1, which showed strong adherence to physical adsorption. The lateral interaction term (α) has positive values (1.9144 and 2.2221), suggesting an attractive behavior of the inhibitor on the Al surface. Parameter x has positive values of 0.8554 and 1.9057, which showed that the adsorbed phytochemicals of the Ricinus communis extract were bulky.
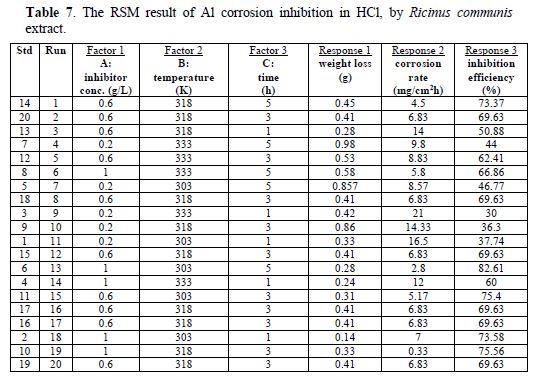
RSM result of the corrosion inhibition process
table 7 showed that aluminum weight loss, corrosion rate and inhibition efficiency were dependent on concentration, temperature and time. The inhibition efficiency increased with higher extract (inhibitor) concentrations. The analysis of the Ricinus communis extract inhibition efficiency is presented in Fig. 2. The plot of predicted versus actual inhibition efficiency was used to test the model significance. The predicted versus actual plot gave a linear graph. The graph (3-D surface plot) showed the relationship between the factors and response of the designed experiment. It revealed that the inhibition efficiency increased with higher concentrations, but decreased with an increase in temperature. A quadratic model describing the relationship between the inhibition efficiency and concentration, temperature and time factors is presented by Equation (14).
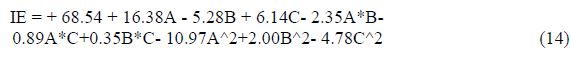
The inhibition efficiency is a function of the inhibitor concentration (C, g/L), temperature (T, K) and time (t, hr). The positive signs in the model signified a synergistic effect, while the negative signs signified an antagonistic effect. The highest power of at least one of the variables was 2, which showed that the mathematical model is a quadratic equation.
Conclusion
The results analysis showed that Ricinus communis extract contained phytochemicals of alkaloids, cardiac glycosides, flavonoids, phenolics, phytates, saponins and tanins. The inhibition efficiency increased with higher extract concentrations, but decreased with an increase in temperature. The free energy of adsorption (?Gads) values were lower than the threshold value of -40 kJ/mol. From the considered isotherms, the R2 values were close to 1, which showed strong adherence to physical adsorption. The activation energy was lower than the threshold value of 80 kJ/mol required for chemisorption. A quadratic model adequately described the relationship between the inhibition efficiency and the inhibitor concentration, temperature and time factors. The thermometric and gravimetric techniques recorded high inhibition efficiencies of 83.93% and 82.61%, respectively, showing that Ricinus communis extract can be employed to control aluminum corrosion in an HCl medium.
References
- Sikandar KS, Tasveer ZB, Kanwal N, Syed AG, Shahana UK. J. Pharm., 4(7) (2013) 88-86.
- Mello LD, Kubota LT. Latin American Applied Research, 44 (2014) 325-329.
- Azari B, Siami A, Ebrahimzadeh MA, Khan BA. Latin American Applied Research, 45 (2015) 139-144.
- Refat MH, Ishaq AZ. Materials, 6 (2013) 2430 – 2451.
- El Maghraby AA. The Open Corrosion Journal, 2 (2009) 189 – 196.
- Kotz JC, Treichel P. Chemistry and Chemical Reactivity, 3rd edition, Harcourt Brace Col. Publishers, New York, 1996.
- Aggarwal OP. Engineering Chemistry, Kahanna Publishers, New Delhi, 3rd, 2010.
- Li X, Deng S. Corrosion Science 65 (2012) 299 – 308
- Loto CA, Popoola API. Canadian of Pure and Applied Sc. 6, (2) (2012) 1973 – 1980.
- Marcano L, Asenawa HD. Agronomy Journal, 83 (1991) 445-452.
- Mayuri PN. Journal of Current Pharmaceutical Research 10 (1) (2012) 19-219
- Mabrouk EM, Shokry H, Abu Al-Naja KM. Met. Alloys, 4, (2011) 98-106
- Eddy NO, Ita BI, Dodo SN, Paul ED. Green Chemistry Letters and Reviews, 5:1, (2012) 43-53.
- Omotioma M, Onukwuli OD. J. Chem. Sci., 14(1) (2016) 103-127.
- Octave L. Chemical Reaction Engineering, Third Edition, John Wiley and Sons, New York (2003).
- Orubite-Okorosaye K, Oforka NC. J. Appl. Sc. Environ. Mgt., 8(1) (2004) 57-61.
- Nwabanne JT, Okafor VN. JETEAS, 2(4) (2011) 619-625.
- Nnanna LA, Owate IO, Nwadiuko OC, Ekekwe ND, Oji WJ. Int. J. of Mat. and Chem., 3(1) (2013) 10-16.
- Alinno IJ, Ejikeme PM. American Chemical Sc. J. 2(4) (2012) 122-135.
- Patel NS, Jauhariand S, Mehta GN, Al-Deyeb SS, Warad I, Hammouti B. Int. J. Electrochem. Sc., 8 (2013) 2635-2655.
Received October 22, 2017; accepted February 08, 2018
* Corresponding author. E-mail address: omorchem@yahoo.com














