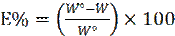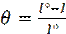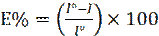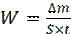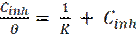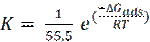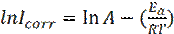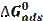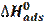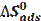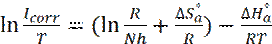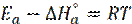Introduction
Different methods have been used to overcome the corrosion problems in the oil and petrochemical industry, either by the selection of suitable materials and/or by changing the environment to make it less aggressive [1]. In the industry, acid solutions are widely used in pickling, cleaning, descaling and oil well acidizing. [2]. In acidic media, corrosion inhibitors play an important role in reducing the corrosion rates of metallic materials [3-8]. They are added in a small amount to slow down or resist the corrosion by the mechanism of adsorption [9 and 10].
Different inhibitors have been used in order to inhibit the corrosion; it was found that the best inhibitors are those that have π electron donation (usually enhanced by the presence of heteroatoms in the aromatic compound). Also, other inhibitors have been extracted from natural sources and used as inhibitor compounds [11-14].
The use of these natural products, such as extracted compounds from leaves or seeds as corrosion inhibitors, has been widely reported by several authors. Essential oils and plants extracts are used as good inhibitors for mild steel corrosion in acidic media [15- 27].
Tribulus Terrestris is a natural herb used for treating many diseases like hypertension [28]. It is a member of the Zygophyllaceae family and it is found in different countries [29]. Tribulus terrestris is also known as Puncture Vine, being used in different medical applications [30, 31, and 32].
This study is aimed to investigate the corrosion inhibition and adsorption properties of different extracts of Tribulus terrestris for mild steel corrosion in HCl, using a potentiostatic technique and gravimetric measurements.
Experimental
Plant Extraction
Tribulus terrestris’s leaves and seeds were collected from sa’adh in the North of Yemen, in July 2010. They were dried at room temperature for three weeks, and then used for immersion extraction and soxhlet extraction.
By using classical Soxhle,t there were obtained both hexanic and ethanolic extracts from 100 g of aerial parts during 8h, using 700 mL of each solvent. These two organic extracts were concentrated and finally stored at +4 °C in well-filled, tightly closed glass vials wrapped in aluminum foil to avoid exposure to light and oxygen [33- 35].
The electrodes which were used in this study were mild steel with a chemical composition of 0.370% C, 0.230% Si, 0.680% Mn, 0.016% S, 0.077% Cr, 0.011% Ti, 0.059% Ni, 0.009% Co and 0.160% Cu. For all the experiments, the surface was cleaned and prepared by using abrasive paper SiC (grades 400, 600, 1200 and 2000), washed with double distilled water. The acidic solutions (1 M HCl) were prepared by dilution of an analytical reagent 37% HCl grade with double distilled water.
GC-MS analysis
By using GC-MS, about 10 µL of Tribulus terrestris extract in a mixture with methanol, chloroform and n-hexane were analyzed using Ultra Trace GC (Thermo-Fisher Scientific) equipped with: i) a Polaris Q Thermo-Fisher Scientific as mass spectra detector; and ii) a VP-5 capillary fused silica column (30 m, 250 µm, 25 µm film thickness). The temperature was kept at 60 °C for 2 min, and then programmed with the rate of 16 °C/min to reach 280 °C in 20 min. Additional operating conditions were the following: He (99.99 %) was used as a carrier gas; 76 kPa was the inlet pressure; the linear velocity was 20cm/s; the injector temperature was 220 °C; the detector temperature was 300 °C; and 1:25 was the split ratio. The identification of the components was based on a comparison of their mass spectra with those of Wiley and NBS Libraries [36] and those described by Adams [37].
Weight loss measurements
Weight loss experiments were carried out in a glass cell with 50 mL of corrosive media, with and without the optimum concentration (1g/L) of Tribulus terrestris extracts.
The samples were tested during 1 week (168h) at room temperature. Finally, the samples were washed in double distilled water and dried in the oven at 70 °C for one hour, and then they were cooled for one hour and weighted. According to the weight loss results, the experiments were repeated to ensure the results. The inhibition efficiency calculated depended on the following equation:
where W and W° are the corrosion rate of mild steel specimens obtained in hydrochloric acid, with and without plant extracts, respectively.
Polarization measurements
A three-electrode cylindrical glass cell full with 100 mL was used to carry out all the electrochemical tests at room temperature. The mild steel specimens were coated with resin, keeping a surface area of 1 cm², and used as working electrode; a saturated calomel electrode as reference electrode and platinum as counter electrode were used. The potentiodynamic curves of mild steel in a HCl solution, in the absence and presence of Tribulus terrestris extracts, were obtained in the potential range from -1 to +0.2 V. The tests of polarization measurements were run with a Voltalab 301 PGZ potentiostat connected with a PC computer and Voltamaster 4.0 software to collect and evaluate the experimental data.
The inhibition efficiency (E %) and degrees of surface coverage (θ) were calculated using the following equations:
where I° and I are the corrosion current densities, with and without the plant extract in a 1 M HCl solution, respectively.
Results and discussion
Extract analysis
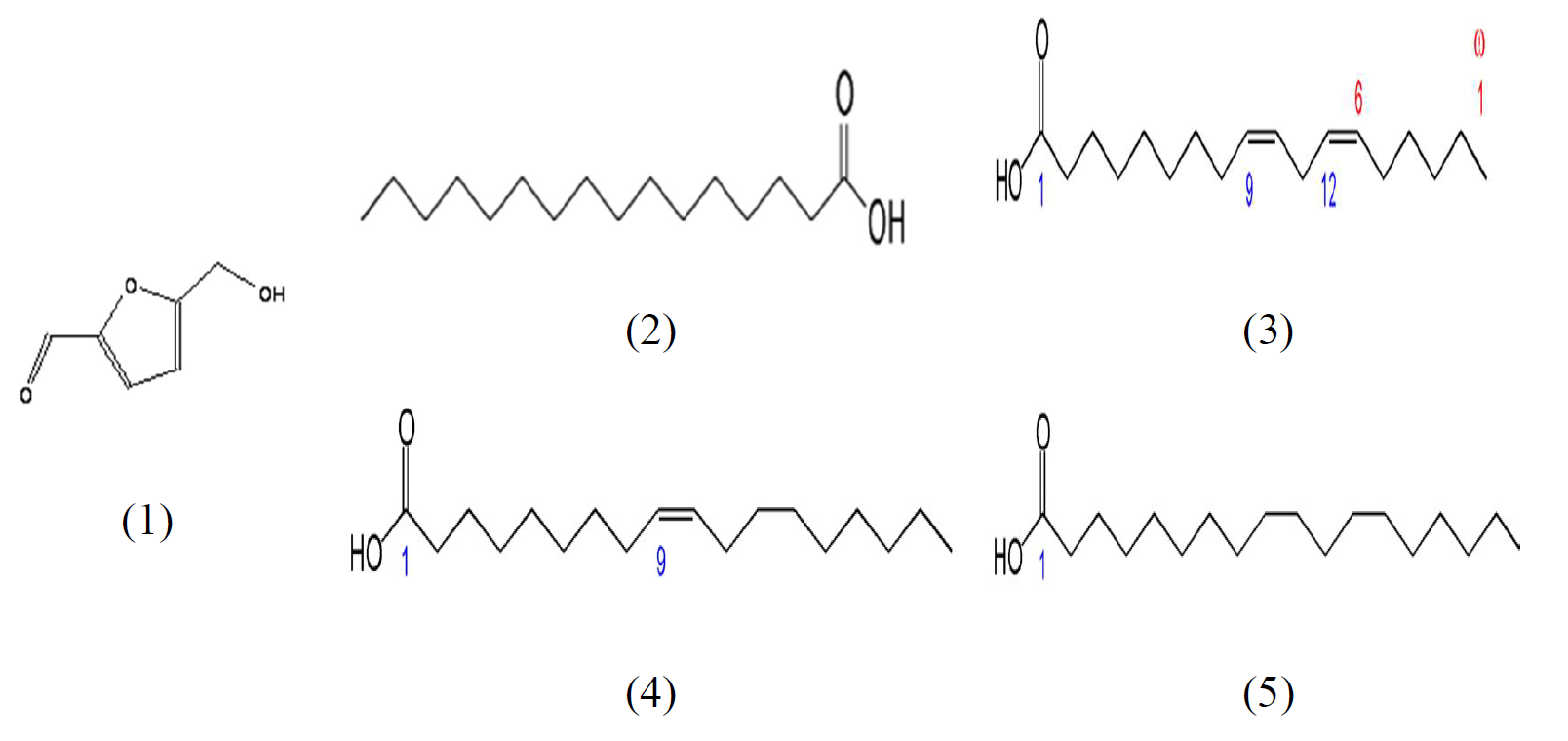
The extracts of Tribulus terrestris were obtained by soxhlet, for the investigation. They had a green color and odor at room temperature, and their yield was found to be 3.27%, 7.59% and 10.66%, for hexanic, ethanolic and aqueous extracts of Tribulus terrestris, respectively. The composition of the ethanolic and hexanic extracts was determined by gas chromatography-mass spectrometry on the basis of the GC retention times, as summarized in Tables 1 and 2. The structures of the major compounds are presented in Fig. 1.
Table 1 Chemical composition of Tribulus terrestris. Ethanolic ectract.
| Compound | Amount % M | RT (min) |
|---|---|---|
| Hydroxy methyl furfural | trace | 8.81 |
| Palmitic acid | 13.43 | 12.23 |
| Linoleic acid | 46.27 | 14.33 |
| Oleic acid | 31.34 | 18.53 |
| Steric acid | 5.97 | 21.34 |
Table 2 Chemical composition of Tribulus terrestris. Hexanolic ectract.
| Compound | Amount % M | RT (min) |
|---|---|---|
| Thujone | trace | 8.96 |
| Hydroxy methyl furfural | 58.69 | 13,78 |
| Palmitic acid | 4.85 | 29,21 |
| Linoleic acid | 3.56 | 32,41 |
| Oleic acid | 29.20 | 32,62 |
| Steric acid | 1.07 | 33,55 |
Weight loss measurements
The inhibition efficiency and corrosion rate of mild steel in one molar hydrochloric acid solution, with and without Tribulus terrestris extracts, are shown in Table 3. These results indicated that the inhibition efficiency for mild steel in one molar hydrochloric with 1.2 g/l of plant extracts at room temperature are 78.56%, 91.71% and 70.52% for the ethanolic, hexanoic and aqueous extracts of Tribulus terrestris, respectively. The corrosion rate (W) and the inhibition efficiency (E%) were calculated using equations 4 and 5 [38]:
where W and W° are the corrosion rate of mild steel, with and without plant extracts, Δm is the weight loss, S is the surface area of the specimens and t is the immersion time of the specimens in the test solution.
Table 3 Parameters of corrosion taken from weight loss measurements of mild steel, with and without the optimum concentration of different extracts of Tribulus terrestris.
| HCl 1M | Ethanolic extract | With hexanoic extract | With aqueous extract | |
|---|---|---|---|---|
| Surface (cm²) | 2.80 | 2.83 | 2.90 | 2.90 |
| weight loss(g) | 0.0989 | 0.0309 | 0.0086 | 0.0227 |
| Time (h) | 168 | 168 | 168 | 168 |
| W (g.cm-².h-1) | 3,61×10-04 | 1,06×10-04 | 3×10-05 | 7,74×10-05 |
| IE (%) | - | 69.83 | 91.39 | 77.83 |
Tafel polarization curves
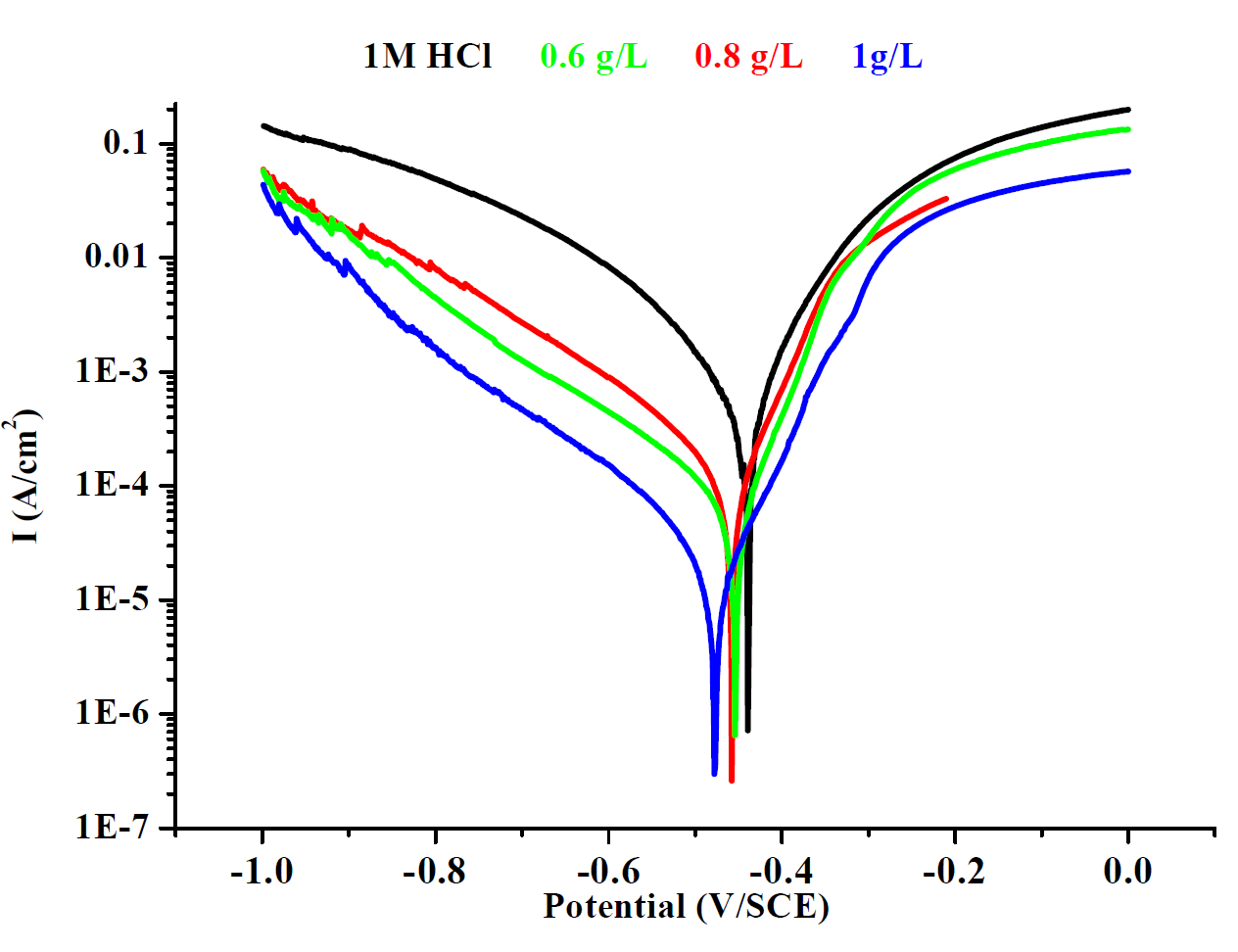
Polarization curves of mild steel, with and without different concentrations of ethanolic, hexanic, and queues extracts of Tribulus terrestris, are shown in Figs. 2, 3 and 4.
The extrapolation of Tafel straight line allowed the calculation of the corrosion (Icorr). The values of current density of corrosion (Icorr), the corrosion potential (Ecorr) and inhibition efficiency (E %) are given in Table 4 for all extracts. The inhibition efficiency (E %) was calculated using the equation (3) [39].
The cathodic ends show the hydrogen evolution reaction, but the anodic ends represent the iron dissolution reaction. [40 and 41]:
As shown in Figs. 2, 3 and 4, both the anodic and cathodic current densities of all extracts were decreased with higher concentrations of plant extracts. It was shown that the ethanolic, hexanic and aqueous extracts of Tribulus terrestris reduced both the anodic and cathodic reactions by adsorption of the extract molecules onto the surface of mild steel. This means that the extracts of Tribulus terrestris act as a mixed type corrosion inhibitor for mild steel in a 1 M HCl solution. Generally, the modes of the inhibition effect of inhibitors are classified into three categories [42 and 43].
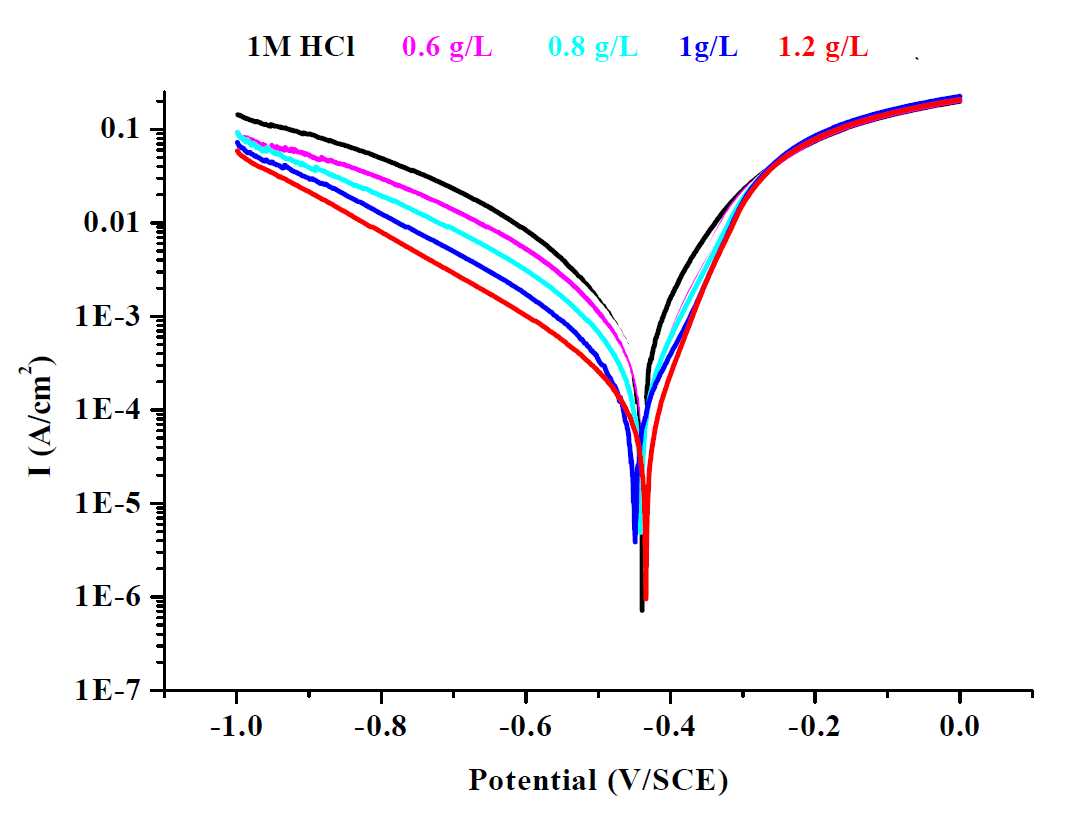
Figure 2 Tafel polarizations curves of mild steel in one molar hydrochloric acid, in the absence and presence of different concentration of ethanolic extract.
Adsorption isotherm behaviour
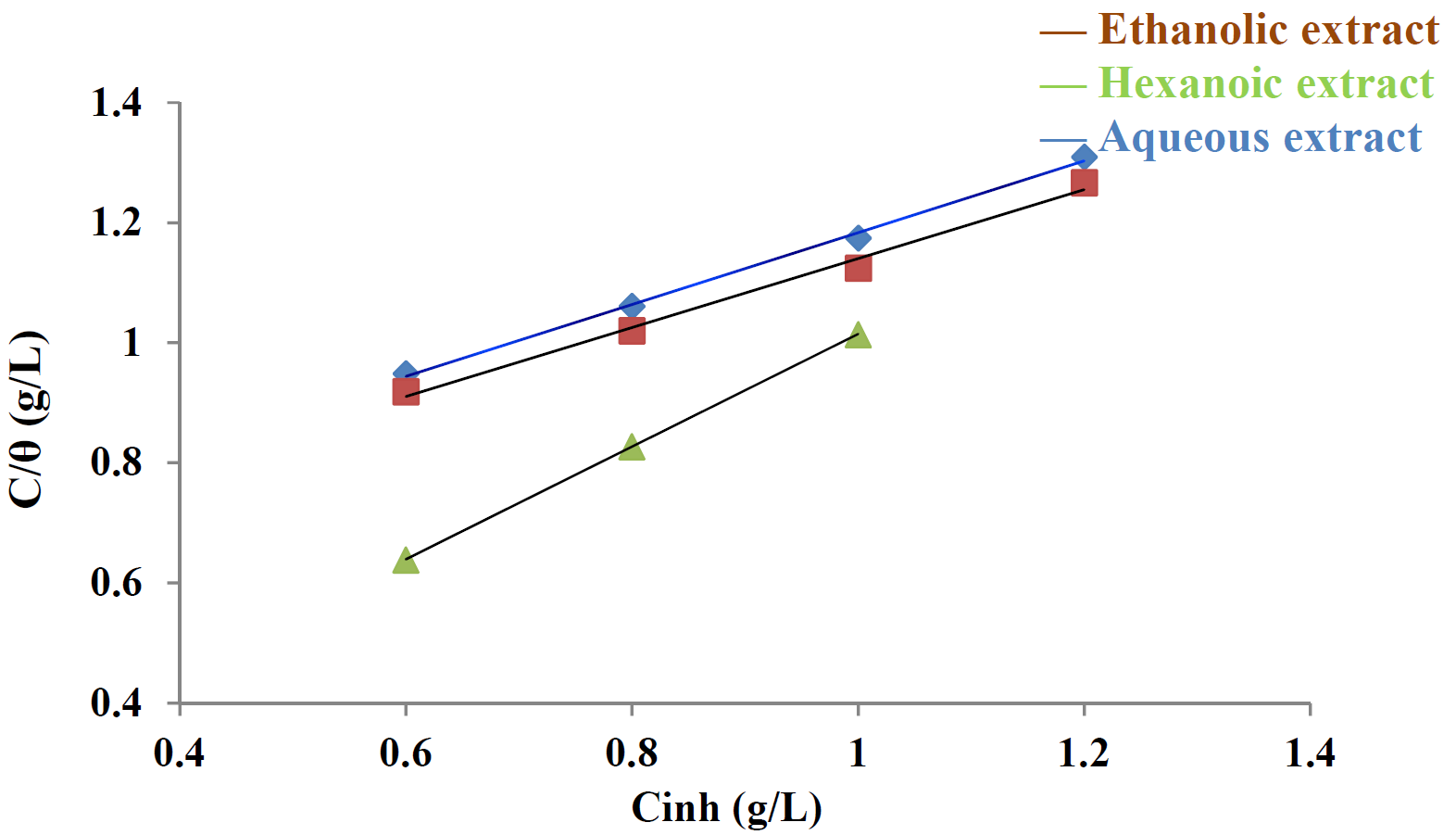
The corrosion inhibition has been explained by the adsorption characteristics of the inhibitor onto the metal surface. In water solutions, in the presence of inhibitor molecules, the metallic surface gets covered with adsorbed water and inhibitor molecules. The interaction between the inhibitor molecules and the metallic surface was studied by a correlation between the surface coverage (θ) and the concentration of inhibitor (Cinh) in the solution, which can be represented by the following Langmuir equation.
where K is the adsorption constant and (θ) is the surface coverage values which were obtained from polarization measurements for various concentrations, and are given in Table 4. The correlation coefficients (R2) were used to choose the adsorption isotherm.
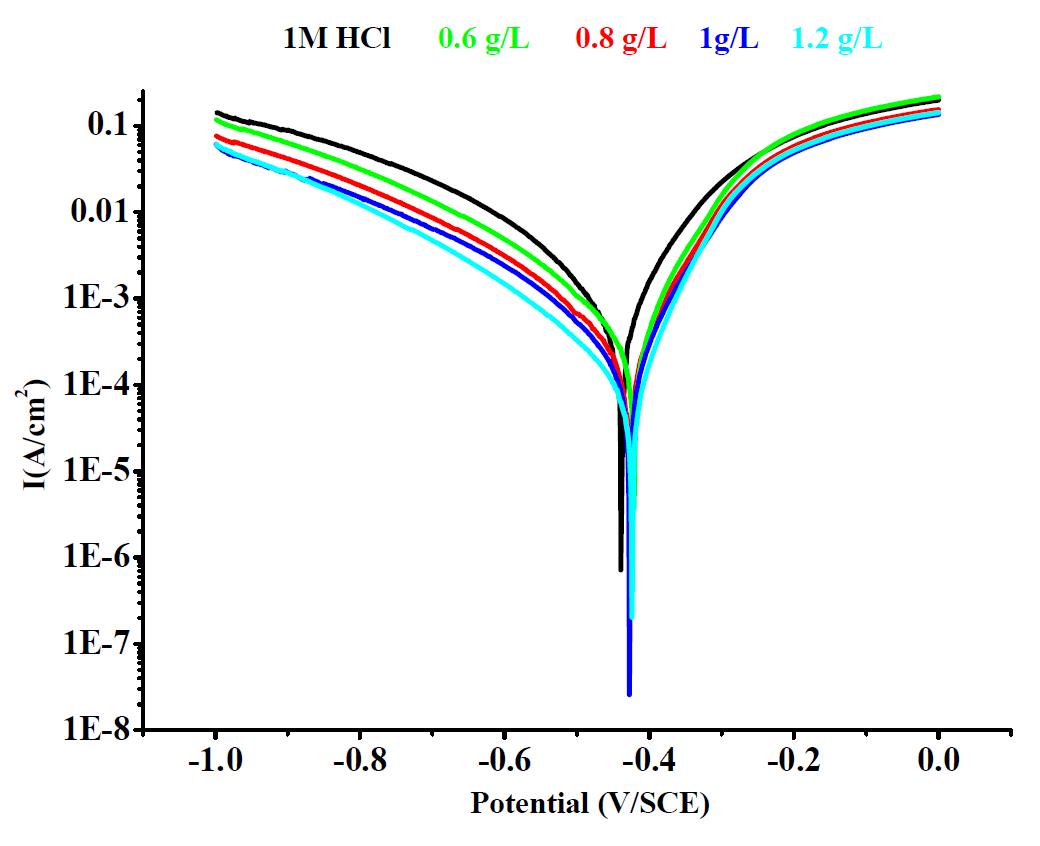
Figure 4 Tafel polarizations curves of mild steel in one molar hydrochloric acid, in the absence and presence of different concentration of aqueous extract.
Table 4 Electrochemical parameters of mild steel at various concentrations of ethanolic, hexanic and aqueous extracts in 1M HCl.
| extracts | C (g/L) | Ecorr mV (SCE) | Icorr (mA cm-2) | Θ | E (%) |
|---|---|---|---|---|---|
| blank | - | -433 | 3,6 | - | - |
| ethanolic extract | 0,6 | -433 | 1,25 | 0.6528 | 65.28 |
| 0,8 | -433 | 0,8 | 0.7840 | 78.40 | |
| 1 | -437 | 0,4 | 0.8890 | 88,90 | |
| 1.2 | -470 | 0,2 | 0.9470 | 94.70 | |
| hexanic extract | 0,6 | -438 | 0,22 | 0.9389 | 93.89 |
| 0,8 | -438 | 0,12 | 0.9678 | 96.78 | |
| 1 | -472 | 0,04 | 0.9881 | 98.81 | |
| aqueous extract | 0,6 | -426 | 1,36 | 0.6324 | 63.24 |
| 0,8 | -426 | 0,91 | 0.7540 | 75.40 | |
| 1 | -426 | 0,55 | 0.8514 | 85.14 | |
| 1.2 | -426 | 0,31 | 0.9162 | 91.62 |
The experimental data indicated that the adsorption of Tribulus terrestris extracts onto the mild steel surface followed the Langmuir adsorption isotherm (Fig .5).
The resulted data suggest that the extracts get adsorbed onto the steel surface at all studied temperatures. Also, the results showed that the corrosion rates increased with higher temperatures in the absence and presence of inhibitor in 1 M HCl solutions. Table 4 showed that the corrosion rate increased with higher temperatures for all experiments, with and without plant extracts, while the inhibition efficiency was decreased; this may be attributed to the weakening of physical adsorption of the inhibitor onto the metal surface with temperatures.
The adsorption constant (K) is related to the standard free energy of adsorption (- ΔGads), as in the following equation [44]:
where (- ΔGads) is the standard free energy of adsorption, 55.5 is the water concentration of the solution in mol/l, R is the universal gas constant and t is the absolute temperature. The negative values of (- ΔGads) indicate the spontaneity of the adsorption process and the stability of the adsorbed layer on the mild steel surface [45]. The values of (- ΔGads) were calculated, and are given in Table 5.
Calculations of activation energy
The activation energies (Ea) of mild steel corrosion, with and without different extracts of Tribulus terrestris, were calculated by the following Arrhenius equation [46]:
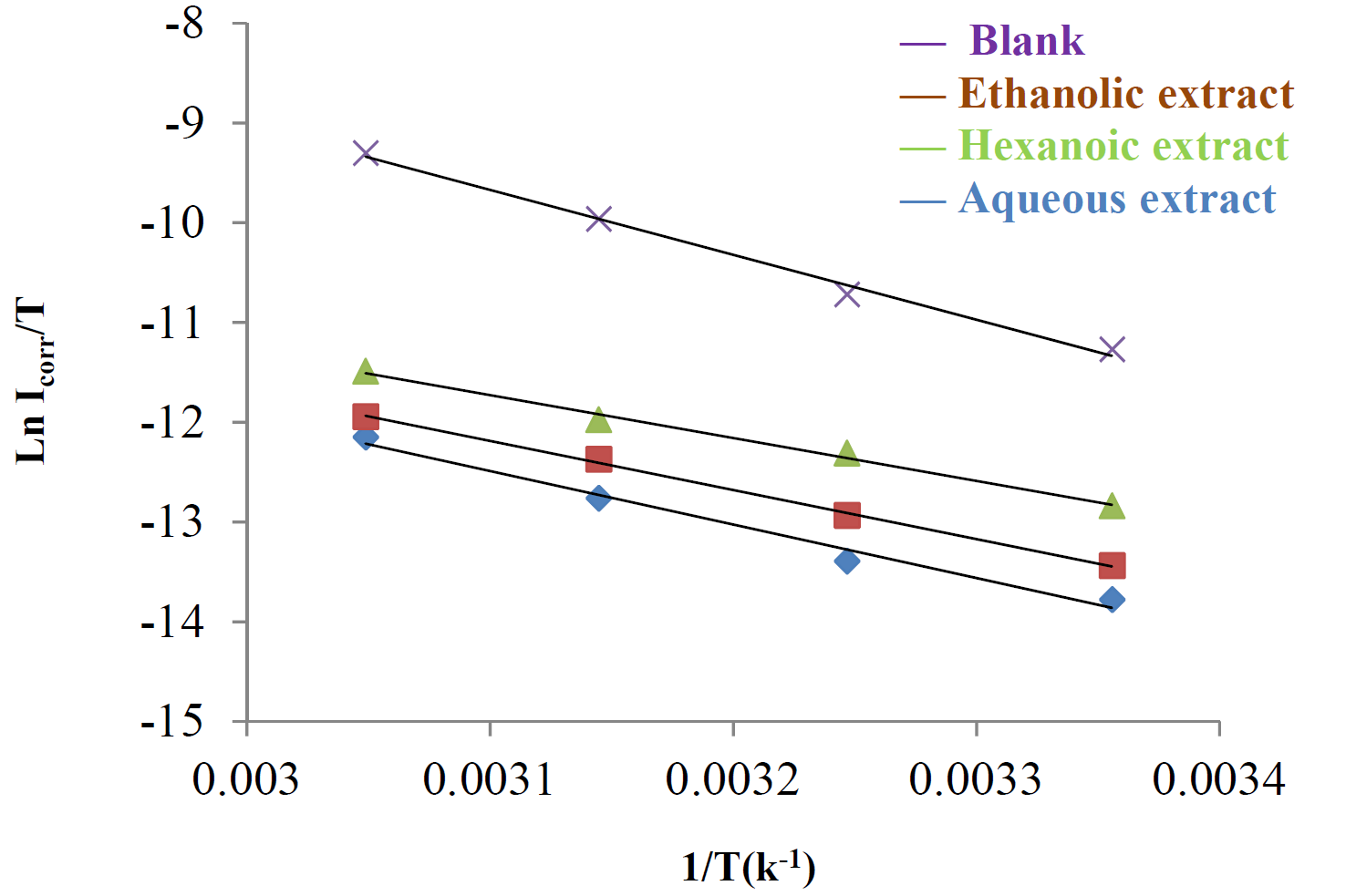
where A is the Arrhenius factor, Ea is the activation energy and Icorr is the corrosion courant. Arrhenius plots for the corrosion rate are shown in Fig. 6. The values of activation energy for mild steel in 1 M HCl, in the absence and presence of different extracts of Tribulus terrestris, were calculated from the slope of (ln Icorr) versus with 1/T plots, and are given in Table 5.
Table 5 Thermodynamic and equilibrium adsorption parameters for the adsorption of plant extracts onto the mild steel surface in 1 M HCl solutions.
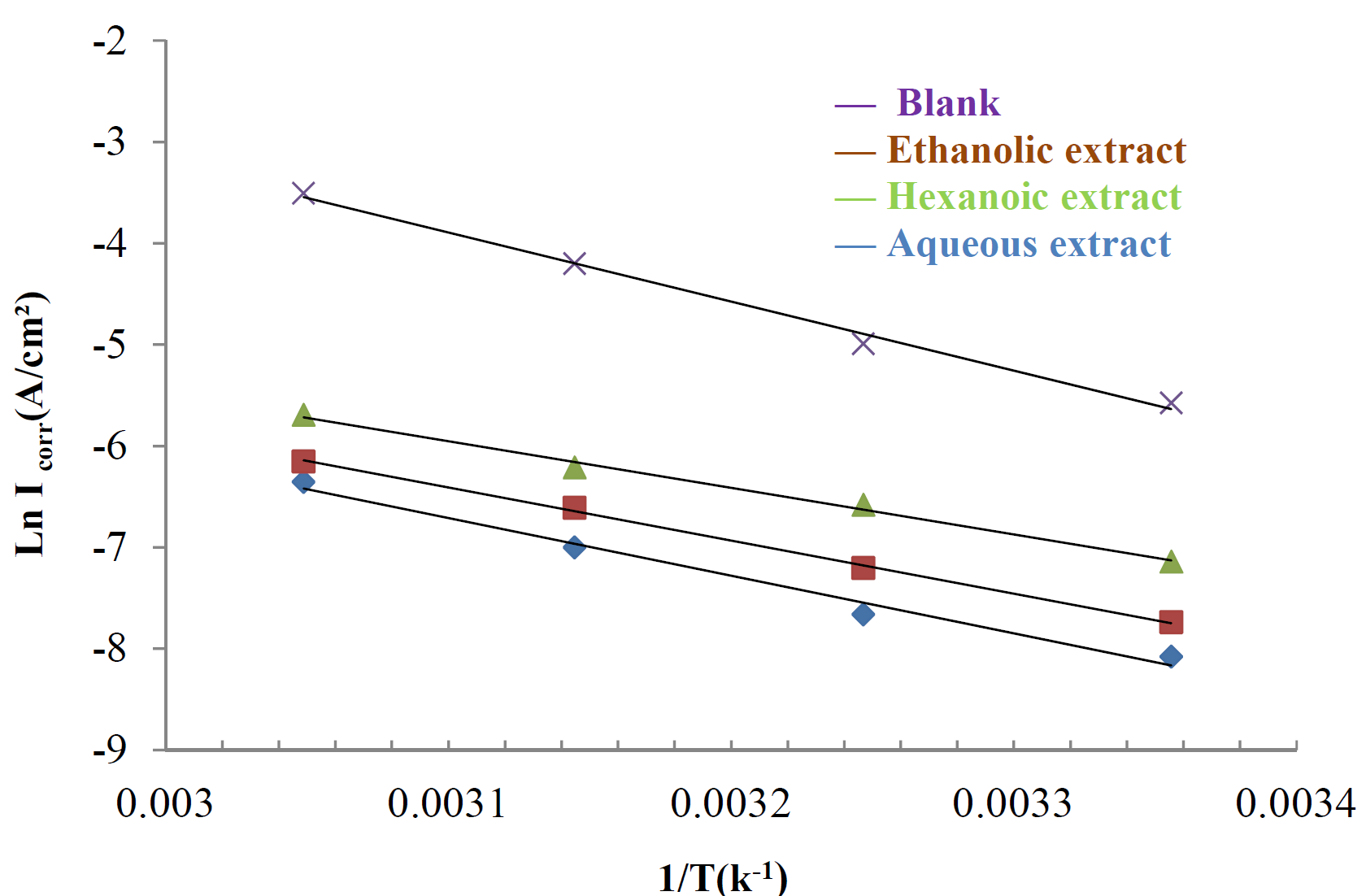
Figure 6 Arrhenius plots of In Icorr against 1/T for mild steel in 1 M HCl, with and without optimal concentrations (1.2 g/L) of different Tribulus terrestris extracts.
The enthalpy (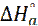 ) and the entropy (
) and the entropy (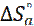 ) of mild steel corrosion in HCl were calculated using the following equation [46]:
) of mild steel corrosion in HCl were calculated using the following equation [46]:
where N is the Avogadro’s number and h is the Planck’s constant. The plot of In(Icorr/T) against 1/T was shown in Fig. 7 with a slope of (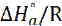 ) and the intercepts-axis of (
) and the intercepts-axis of (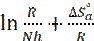 ). The values of
). The values of 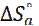 were calculated and given in Table 5. All values of Ea are larger than the values of
were calculated and given in Table 5. All values of Ea are larger than the values of 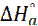 , which indicated that the corrosion process must involve a hydrogen evolution reaction, associated with a decrease in the total reaction volume [45].
, which indicated that the corrosion process must involve a hydrogen evolution reaction, associated with a decrease in the total reaction volume [45].
The difference value of the Ea - 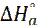 is 2.60 kJ/mol, which is approximately equal to the value of RT (2.63 kJ/mol). These values indicated that the corrosion process is a molecular reaction, as it is defined by the following equation [47]:
is 2.60 kJ/mol, which is approximately equal to the value of RT (2.63 kJ/mol). These values indicated that the corrosion process is a molecular reaction, as it is defined by the following equation [47]:
The positive values of the enthalpies show the endothermic nature of the steel dissolution process and the activated complex in the rate determining step, which was cleared by the negative entropies values, showing an association instead of a dissociation step [48 and 49].
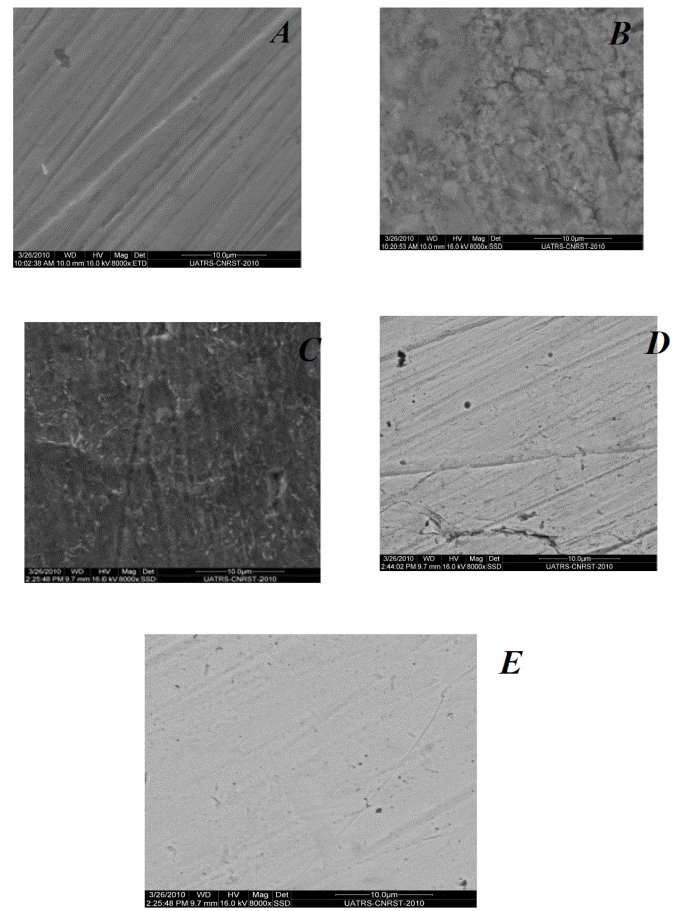
SEM studies
Scanning electron microscopy (SEM) micrograms of mild steel specimens immersed for one week in 1 M HCl solutions, with and without 1.2g/L of Tribulus terrestris extracts, are shown in Fig. 8.
In presence of the extract, the comparative morphologies show a smooth surface of mild steel specimens with deposited extract on it; on the other side, a rough surface of mild steel is clear in the absence of the extract [50].
This result confirmed the results of electrochemical techniques and indicated that the extract of Tribulus terrestris inhibited mild steel corrosion by the adsorption of extract molecules onto the mild steel surface.
Conclusions
The inhibition efficiency of Tribulus terrestris extracts for mild steel corrosion in 1 M HCl media increases with higher concentrations of Tribulus terrestris extracts, and decreases with the rise in temperature. The potentiodynamic polarization measurements show that Tribulus terrestris acts as a mixed type inhibitor. Isotherm observations indicated that the extract molecules adsorbed onto the mild steel surface, obeying Langmuir adsorption isotherm.
SEM studies confirm that the adsorption of the extract onto the mild steel surface inhibited the corrosion, which emphasizes the results of electrochemical technique.