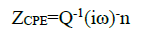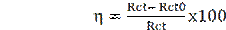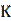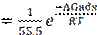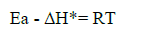Introduction
Combating metal corrosion in acidic media is a long-standing effort of scientists and engineers. Due to the corrosive effect of these acids and to the huge economic loss that is suffered by the concerned industries resulting from rapid corrosion of metallic parts, many chemical compounds have been used to reduce and control this process. Suitable inorganic and organic inhibitors have shown good anticorrosive activity (1, 2). Many of these compounds have proved to be highly efficient in corrosion inhibition, but their use is still limited, due to their toxicity and ecological risks (3). Because of this, attention has been drawn towards the use of natural products as corrosion inhibitors, due to their biodegradability and low cost (4). This advantage gives them the opportunity to replace the expensive and toxic chemical inhibitors. The corrosion inhibition abilities of different plant extracts differ from one type to another, depending on the used part of the plant and of its location (5). Some plant extracts have been studied as effective steel corrosion inhibitors in HCl and H2SO4 solutions, as reported in (6-11). The present work reports the inhibition effect of Sumac leaf extract, Rhus Coriaria (RC), and its chemical constituent, Quercetin, on mild steel corrosion, in 0.5 M HCl and 0.5 M H2SO4 solutions. The difference in the inhibition effect of RC plant extract with Quercetin is herein discussed. It is expected to provide a link between the RC inhibition effect and that of its chemical constituent, Quercetin, on mild steel corrosion in acidic solutions. The novelty of this study is that it sheds some light on the effect and interaction of the acidic anion with the plant extract (RC) and Quercetin, and on its contribution to mild steel corrosion inhibition. Thermodynamic studies provide a great link on the effect of the acidic anion and its correlation with mild steel corrosion retarding.
Experimental
Solution preparation
The tested solutions were prepared using distilled water and analytical grade reagents, HCl and H2SO4, obtained from BDH chemical company. Quercetin with ≥ 95% purity was obtained from Sigma Aldrich. The plant leaf was dried for 2 h in an oven at 80 ºC, after which it was grinded into powdery form, from which stock solutions of plant extracts were prepared. A mass of 10 g of the grinded powder plant leaf was refluxed in 100 mL of distilled water for 2 h, and then filtered to remove any contamination. From the filtrate, a volume of 10 mL was evaporated, and the obtained residue was weighed and further used in the preparation of the stock solution concentration. Different concentrations of the extract, in 0.5 M HCl or 0.5 M H2SO4, were prepared by adding 4 M HCl or 4 M H2SO4 to the required volume of the stock solution of the plant leaf extract, and mixed with double distilled water.
Electrochemical studies
A frequency response analyzer (FRA)/potentiostat supplied from ACM instruments (UK) was used to determine the polarization and electrochemical impedance measurements. The detailed electrochemical techniques were reported in a previous publication (12).
FTIR analysis
The FTIR spectra of RC leaf extract were analyzed at Faculty of Engineering and Physical Sciences, University of Surrey (UK), using a solid FTIR sample holder. The infrared spectra of the solid sample were recorded using a Perkin-Elmer (2000 FTIR) spectrometer, by averaging 32 scans at a resolution of 4 cm-1 in the spectral region between 4000 and 500 cm-1.
AFM surface morphology
Atomic force microscopy (AFM), operated in the contact mode, under ambient conditions, with an Agilent 5420 Atomic Force Microscope, was used to determine the morphology of mild steel surface. Solutions of 0.5 M HCl and 0.5 M H2SO4, with and without different concentrations of both RC leaf extract and Quercetin, were immersed for 2 h, with specimens of mild steel, at 25 (C. After that, exposure images of the specimens were recorded using a probe with a maximum measuring area of 100 x 100 micrometer. AFM analysis was done at Lebanese University-Fanar Campus, Platform for Research in Nanoscience and Nanotechnologies.
Results and discussion
Spectrophotometric analysis
The Lebanese sumac called Rhus Coriaria (RC) contains different chemical compounds. It has a broad range of nutritionally and medicinally significant photochemical components, such as tannins, flavonoids, anthocyanins, organic acids, flavones (such as Myricetin, Quercetin and Kaempferol), proteins, fiber, volatile oils, nitrates and nitrites (13).
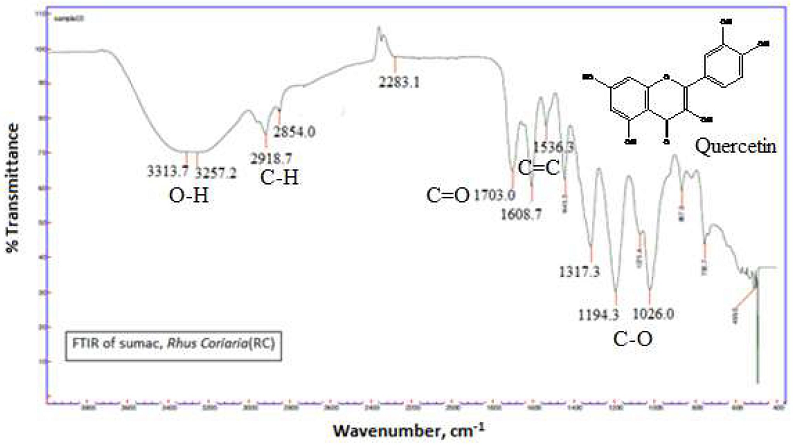
Fig. 1 shows the FTIR spectrum of RC leaf extracts. The absorption bands exhibit C-H (2918.7cm-1), O-H (3313.7- 3257.2 cm-1), C = C (1608.7-15363 cm-1) and C = O (1703 cm-1) stretching vibrations. The strong band around 1194.3-1026.0 cm-1 is assigned to C-O single bond. The obtained IR spectrum of RC leaf extract is in a good agreement with that of the suggested spectrum band of the hydrolyzed form of Quercetin.
Potentiodynamic polarization curves
The potentiodynamic polarization curves of steel in a 0.5 M HCl solution, in the absence and presence of 0.75 g.L-1 of RC and Quercetin, are shown in Fig. 2. A clear shift in both anodic and cathodic parts of the metal dissolution, towards lower values of corrosion current densities, indicates that both RC and Quercetin delay mild steel corrosion. Similar observations were reported for steel in 0.5 M H2SO4, but still with greater inhibition efficiency for the plant extract, up to 84% in HCl media, rather than in sulfuric acid, as reported in Table 1.
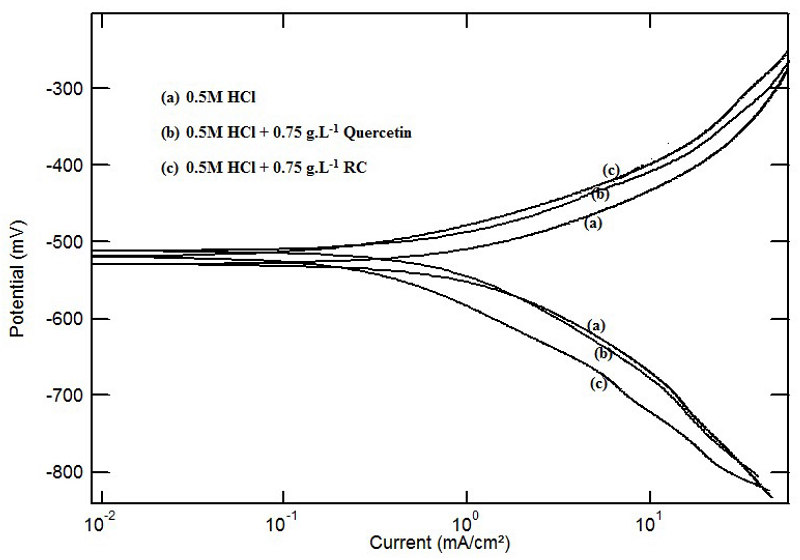
Figure 2 Potentiodynamic polarization curves of steel in 0.5 M HCl, in the absence and presence of 0.75 g.L-1of Quercetin and RC leaf extract, at 30 °C.
The electrochemical corrosion parameters shown in Table 1 were calculated from polarization measurements, along with the percentage of inhibition efficiency,(, where ( = [(i0-i)/i0] x 100, and ioand i are the corrosion current densities in the absence and presence of plant leaf extracts, respectively.
(10, 14) show clearly that corrosion current density (icorr) decreases considerably with the addition of the plant extract and of Quercetin.
The inhibition efficiency, (, increases as the plant extract concentration increases and reaches a maximum efficiency of 84% at 80 ppm (0.8 g/L), being assisted by physical adsorption mechanism onto the metal surface. Further increase in the RC concentration showed no more changes in percentage inhibition, indicating that optimum concentration has been reached. Plant extract concentration greater than 0.8 g/L gave a constant inhibition efficiency or a small decrease in it.
The appearance of critical concentration, after which the extract inhibitive effect decreased, was also observed in other study (15).
Table 1 Electrochemical polarization parameters for mild steel corrosion in 0.5 M HCl and 0.5 M H2SO4, in the absence and presence of different concentrations of RC leaf extract and Quercetin, at 30 ºC.
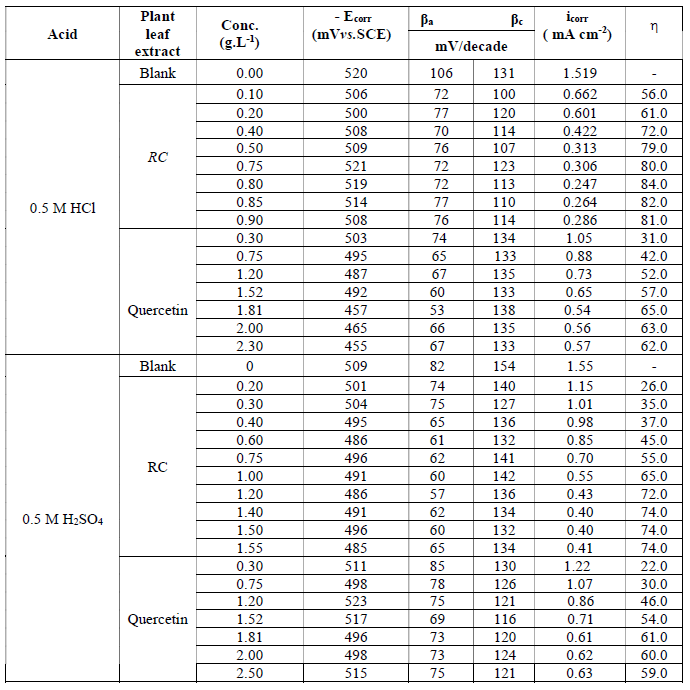
The Tafel slopes, βa and βc, did not change significantly with the inhibitor addition, which indicates that it does not alter the mechanism of hydrogen evolution and metal dissolution process; inhibition action takes place through a similar mechanism (16). The addition of RC leaf extract retards mild steel corrosion much more than Quercetin in both acidic media.
The curves obtained from the plot of degree of surface coverage (( = (/100) with the RC leaf extract concentration and Quercetin in 0.5 M HCl, shown in Fig. 3, indicate that the inhibition process took place through adsorption. The increase in inhibition, with the inhibitor concentration initial rising part of the curve, reveals an increase in the active extract component adsorbed onto the metal surface. This increased inhibitor adsorption leads to the formation of a protective layer that blocks the active sites on the metal surface and shields it from corrosion. At high concentrations, a saturated constant part of the curve is observed, which illustrates the formation of mono-layer films on the metal surface (17). The curve also shows that RC inhibits the corrosion process more effectively than Quercetin in both acidic solutions.
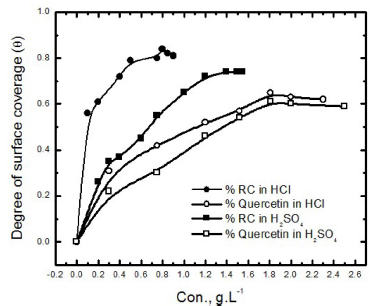
Figure 3 Variations of degree of surface coverage, obtained from polarization measurements, with the RC leaf extract and Quercetin concentrations, in 0.5 M of HCl and 0.5 M of H2SO4.
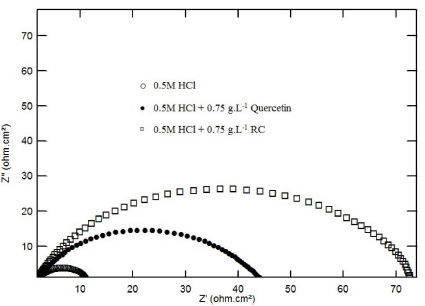
Figure 4 Nyquist plots for mild steel in 0.5 M HCl, in the absence and presence of 0.75g.L-1of RC and of Quercetin, at 30 ºC.
Electrochemical impedance spectroscopy measurements
Steel Nyquist diagram in 0.5 M of HCl and H2SO4 solutions containing 0.75g.L-1 of RC and Quercetin are shown in Fig. 4. The figure exhibits one single depressed semicircle, showing that mild steel corrosion is controlled by charge transfer (16, 17). The similarities between the observed semicircles for both inhibitors, in the presence of acid alone, and of RC or of its chemical constituent, strongly suggest that the mechanism of metal dissolution is not changed by the inhibitor (6). Similar results were obtained for steel Nyquist impedance plot of in 0.5 M H2SO4, in the absence and presence of RC leaf extract and Quercetin.
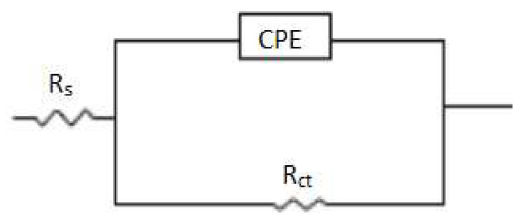
The experimental data obtained from impedance spectra for different Nyquist plots were analyzed by fitting them to a simple equivalent circuit model (Fig. 5), which includes the solution resistance, Rs, and the constant phase elements (CPE), in parallel to the charge transfer resistance element, Rct. (18).
To compensate for non-homogeneity in the system, the capacitances were implemented as a constant phase element (CPE) that is defined by two values, Q and n, which are used to quantify several physical phenomena. The impedance, Z, of CPE is presented by:
where i = (-1)1/2, ω is the frequency in rad s-1, ω = 2(f and f is the frequency in Hz. If n equals 1, then, ZCPE is identical to that of a capacitor, ZC = (iωC)-1, where C is the ideal double layer capacitance. In fact, a capacitor is actually a constant phase element with a constant phase angle of 90. For a non-homogeneous system, n values ranges from 0.9 to 1, and Q represents the non-ideal double layer capacitance.
From impedance measurements, the percentage inhibition efficiency (() was calculated according to the equation (21):
where Rct0 and Rct are the values of the charge transfer resistance (Ω cm2), in the leaf extract absence and presence, respectively.
As it is seen in Table 2, charge transfer resistance (Rct) values increase with an increasing concentration of RC extract and of Quercetin, while Qdl decreases with an increasing concentration, suggesting that the inhibitor functions by adsorption onto the steel surface. RC and Quercetin molecules gradually replace water molecules, which leads to the formation of a protective film onto the metal surface, and thus decrease the extent of the metal dissolution (20). The corrosion inhibition efficiency reached 84%, with 0.75 g.L-1 of RC concentration. The trend in the increase of ( is related to the degree of surface coverage of the inhibitor molecule at the steel surface. The high corrosion inhibition efficiency obtained at optimum concentration, and which decreases with further increase in concentration, indicates the saturation of the mono adsorbed layer at this concentration. This suggests that mild steel corrosion inhibition in a hydrochloric acid solution took place by an adsorption/desorption mechanism. The concentration of both inhibitors, RC and Quercetin, is high enough to support the adsorbed layer formed at the metal surface and to sustain the process of adsorption/desorption almost at equilibrium, but more shifted towards desorption.
Table 2 Impedance parameters for mild steel in 0.5 M HCl and 0.5 M H2SO4 containing different concentrations of RC leaf extract and of Quercetin, respectively, at 30 ºC.
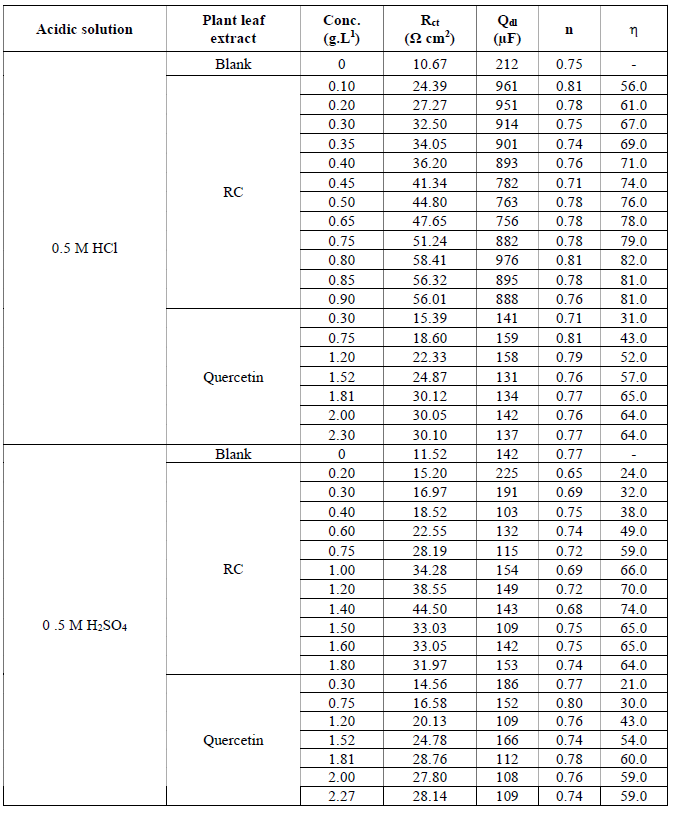
It is supposed that the thickness of the surface film of adsorbed particles within outer Helmholtz layer (OHL) would shrink, while the disperse part of the double electrical layer would expand (21). The inhibition efficiencies obtained from both EIS measurements and potentiodynamic polarization curves are very close, which indicates the validity of the results from both techniques. In addition to this, RC leaf extract is a better corrosion inhibitor in 0.5 M HCl than Quercetin, and, still, HCl is suppressing H2SO4 media, as shown in Fig. 4.
AFM surface morphology
Samples of mild steel immersed in 0.5 M HCl, with and without inhibitor, were studied using atomic force microscope (AFM) to examine their surface morphologies. AFM is best used to identify the surface roughness of the metal samples on a micro scale and to produce 3D images of the surface (22, 23).
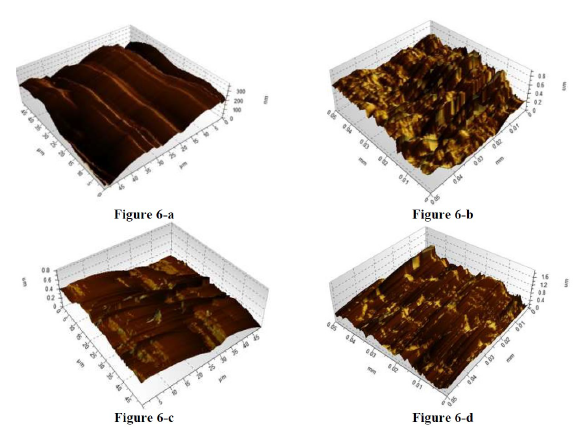
Figure 6 Three-dimensional AFM images for steel. Before immersion: (a) bare metal. And after immersion in: (b) 0.5 M HCl; (c) 0.5 M HCl + 0.75 g.L-1 RC; and (d) 0.5 M HCl + 0.75 g.L-1 of Quercetin, respectively, at 25 ºC, for 2 h.
Fig. 6 shows the 3D images obtained before and after 2 h of metal immersion in a solution with acid alone and with the inhibitor. When mild steel was exposed to a blank HCl solution, metal dissolution rate was observed to be high (6-b), with a surface full of corrosion products, pits and cavities, in comparison to the surface of pure mild steel (Fig. 6-a). However, in the presence of RC leaf extracts and of Quercetin, the surface roughness of the mild steel was seriously reduced, due to the formation of a protective layer which lowered the corrosion rate, indicating the inhibiting effect of both additives.
Precise observation showed a smoother mild steel surface, in the presence of RC leaf extract (6-c), in comparison with Quercetin (6-d), indicating that the plant extract still serves as a better and more efficient retarder than chemical compounds, even if Quercetin is one of its chemical constituents. In addition to that, the surface roughness is much more reduced in HCl than in H2SO4, even though both acids were equal in concentration, which was 0.5 M. This indicates better inhibition efficiency for the plant leaf extract and Quercetin in HCl media, than of that in H2SO4, suggesting the greater role that the acidic anion is playing. The surface morphology results obtained on the addition of RC leaf extract and of Quercetin show that a film of adsorbed layer is responsible for corrosion inhibition, confirming results obtained from electrochemical impedance spectroscopy and potentiodynamic polarization measurements, and display a better inhibiting action of RC leaf extract.
Adsorption considerations
Surface coverage characteristics and the type of adsorption isotherm can play a main role in determining the inhibitor properties. Fitting experimental data into different adsorption isotherms can also provide important information about the inhibition mechanism. Adsorption isotherms can successfully describe the interaction taking place at the surface between the adsorbed inhibitor and the metal (24).
All the obtained values of the surface coverage (θ = ( / 100) were fitted to different adsorption isotherms. According to the obtained correlation coefficients, both RC and Quercetin fitted well thermodynamic-kinetic and Flory- Huggins models. The equations describing Flory-Huggins isotherm and kinetic - thermodynamic model with their parameters are mentioned in detail in our previous work (12). The parameters obtained from linear fitting of the mentioned models for RC leaf extract and Quercetin, in 0.5 M HCl and 0.5 M H2SO4, at 30 ºC, are shown in Table 3.
Table 3 Linear fitting parameters of RC leaf extract and of Quercetin, according to the mentioned models, in 0.5 M HCl, at 30 ºC.
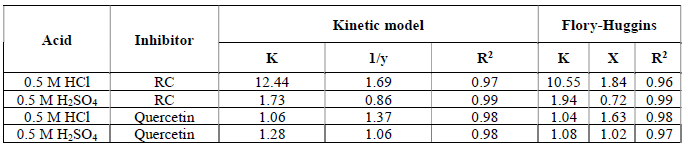
The adsorption parameters for the kinetic-thermodynamics models showed that the values of the number of active sites resided by a single molecule 1/y are bigger than unity, for both RC leaf extract and Quercetin, in 0.5 M HCl and 0.5 M H2SO4, at 30 °C. This indicates that the molecules of the RC leaf extract and of Quercetin fill more than one active site. However, only 1/y for RC in 0.5 M H2SO4 indicates that it occupies less than one active site. This gives a key clue to the fact that the acidic anion is playing an important role in the inhibitor adsorption mechanism onto the metal surface, since values for Quercetin are the same in both acidic media, while for the plant extract (RC) they differ between HCl and H2SO4, which suggests the possible interaction of another chemical ingredient (25). In addition to that, the values of the binding constant, K, which show how stronger or weak an interaction between the inhibitor molecule and the metal is, are the same for Quercetin, in both acidic media, indicating that there is no effect of the acidic anion on this interaction (26). However, when using the plant extract, K is greater in HCl than in H2SO4, indicating the effect of the acidic anion on another chemical ingredient, which could be highly adsorbed and cause this strong interaction. More information was obtained from Flory-Huggins isotherm, and the value of the size parameter, x, which is a measure of the number of adsorbed water molecules substituted by inhibitor molecules, is very close to that obtained from the kinetic model. Therefore, the results obtained for 1/y and x together cause one to think that the inhibitor molecule is so bulky that can occupy more than one active site, when adsorbed onto the metal surface (27).
Effect of temperature
Another valuable factor that could influence the metal corrosion rate, and change the inhibitor adsorption onto the electrode surface, is temperature.
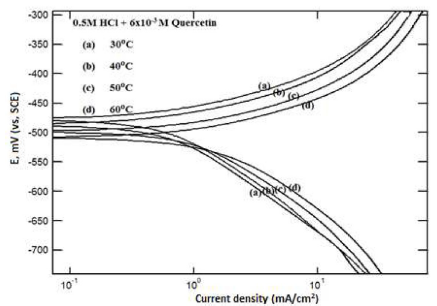
Figure 7 Potentiodynamic polarization curves for mild steel, in 0.5 M HCl, in the presence of 0.75 g.L-1 of Quercetin, at different temperatures.
The curves in Fig. 7 for Quercetin, with mild steel, in the absence and presence of 0.5M HCl, at different temperatures, correspond to the potentiodynamic polarization curves. Similar curves were observed for RC leaf extract and for Quercetin, in 0.5 M H2SO4, respectively.
The inhibition efficiency decreases with increasing temperature, as it is seen in Fig. 7. This is due to the increased rate of mild steel dissolution process, with the contribution of the partial inhibitor desorption from the metal surface, with the increase in temperature (28, 29). Thus, the corrosion rate increases as the desorption of the adsorbed film onto the mild steel surface increases.
Similar observations were obtained for RC leaf extract and for Quercetin, in 0.5 M H2SO4 (Fig. 7 and Fig. 8). The values of the apparent activation energy (Ea), obtained in the inhibitor presence and absence, could give some information about the mechanism of inhibition that took place.
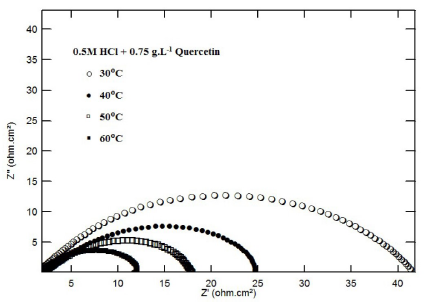
Figure 8 Nyquist impedance plots for mild steel, in 0.5 M HCl, in the presence of 0.75 g.L-1 of Quercetin, at different temperatures.
The thermodynamic parameters, including activation energy, Ea, activation enthalpy, ∆H* a, and the apparent entropy of activation, ΔS*, were computed from Arrhenius equation and from the transition state equation mentioned in (12).
The binding constant, K, was determined from the equation below (30).
where ΔGads is the standard free energy of adsorption, T is the absolute temperature in Kelvin, R is the molar gas constant and 55.5 represent the concentration of water in the solution, expressed in molar.
The calculated values for the activation parameters, together with ΔGads, are given in Table 4. The obtained data show that Ea values increased in the presence of RC extract and of Quercetin, compared to the blank, which indicates physical adsorption, where the chemical species of the extract caused geometric blocking of the metal surface (31). Also, the average value calculated for the difference of Ea -∆H*, for RC and Quercetin. is 2.64 kJ/mol, in 0.5 M HCl and 0.5 M H2SO4, which is very similar to the average value of RT, that is 2.63 kJ/mol, at 30 ºC. This gives the idea that the corrosion process was taking place through a unimolecular reaction, as it is given by the following equation (32):
From Table 4, it is clear that all ΔH* values are positive, which reveals that the formation process of the activated complex is endothermic. Also, the magnitude of the enthalpy is lower than 80 kJ/mol, indicating a physical adsorption process, since the enthalpy of chemisorption process approaches 100 kJ/mol. The negative ΔS* values (A = (r2 complex) in the rate determining step represent an association step rather than a dissociation step. This gives rise to the fact that more orderings taking place on going from reactants to the activated complex (33-35).
Table 4 The activation parameters of mild steel in 0.5 M HCl, in the absence and presence of 0.75 g.L-1 of RC leaf extracts and of Quercetin, respectively.
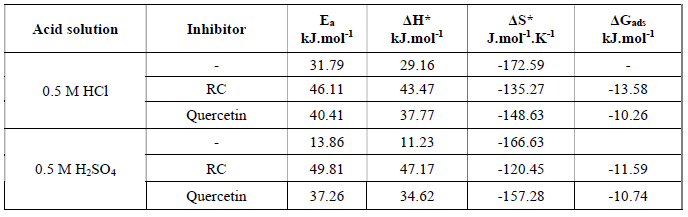
The process of adsorption of RC leaf extract and of Quercetin was spontaneous, as shown by ΔGads negative values. Also, these values indicate the stability of the adsorbed layers onto the mild steel surface, in the 0.5 M HCl and 0.5 M H2SO4 solutions. Generally, the physisorption mechanism, which is an electrostatic interaction between the charged molecules and the metal surface, corresponds to ΔGads values up to -20 kJ.mol-1. However, chemisorption mechanism, which could be the sharing or transfer of an electron pair or π electrons, from the inhibitor molecule to the metal surface, to form a coordinate bond, represents values around -40kJ moL-1 or more negative (31, 36). The obtained results suggest that the adsorption of RC leaf extract and of Quercetin onto the metal surface is physisorption rather than chemisorption
Conclusions
The results presented in this paper show that Rhus Coriaria (RC) leaf extracts inhibit mild steel corrosion in a HCl solution, more than in a H2SO4 solution, and leaf extracts were more promising than active chemical components in both acidic media.
Also, the inhibition efficiencies of the RC leaf extract and of one of its chemical components, Quercetin, increase with higher concentrations and decrease with increasing temperature, suggesting an inhibition mechanism through the formation of a protective film that blocks active corrosive sites on the metal surface, via physical adsorption.
However, the results of AFM surface morphology confirm the formation of a film on the mild steel surface, which is responsible for the decrease in the surface roughness and, in its turn, effectively retards steel corrosion.
Moreover, Flory-Huggins isotherm and kinetic - thermodynamic model indicate that the corrosion process is a unimolecular reaction, and that the acidic anion is playing an important role in the inhibitor adsorption onto the metal surface.
Finally, results from all the employed techniques are in reasonably good agreement, and point to the fact that RC leaf extract is a better inhibitor than Quercetin, within the range of investigated concentrations.
Therefore, the plant extract can be considered as a good source of corrosion inhibitors which are relatively cheap, non-toxic, biodegradable and effective in an acidic medium.