Introduction
Determination of drugs in biological matrices requires the development of reliable analytical methods in clinical, industrial and research contexts. These analyses are essential in pharmacokinetic studies and therapeutic monitoring of drugs [1].
Type II diabetes is a metabolic disorder characterized by hyperglycemia, due to cellular resistance to insulin, combined with insufficient pancreatic secretion of insulin. Over 300 million people suffer from diabetes worldwide [2].
Gliclazide (GLZ) is a second-generation sulfonylurea oral anti-diabetic drug, effective in controlling blood glucose in type II diabetes mellitus. It acts mainly on pancreatic sulfonylurea receptors (SURs), at the surface of β-cells, by increasing the secretion of insulin [3].
In contrast with the first-generation drugs, gliclazide preparations are highly efficient, thus, treatment doses are two to three times lower [4]. Therefore, the development of simple new analytical methods with better specificity and higher sensitivity is very important [5].
Many methods for gliclazide determination in pharmaceuticals are described in literature. An important group consists of UV and visible spectrophotometric methods [6, 7].
Chromatographic methods, including liquid chromatography [8-10], thin-layer chromatography [11, 12] and gas chromatography [13], were also proven to be useful.
Comparatively, only few reports dealing with the electrochemical behavior of gliclazide and its electro-analytical determination using voltammetry [14-16] were published.
There are some advantages of the electrochemical methods, including simplicity, low cost, high sensitivity and miniaturization [17]. The development of screen-printed electrodes (SPEs) has become a major revolution in the construction of electrochemical sensors/biosensors [18]. SPEs have been designed especially for miniaturization of electrochemical analytical systems [19].
SPEs are highly-versatile, easy to use, cost-effective analytical tools and also suitable to miniaturization [20]. Furthermore, unlike conventional electrodes, such as glassy carbon electrode, SPEs avoid the cleaning process [21].
Nowadays, the development of new materials capable to change the electrode surface with better analytical properties, including graphene, nanoparticles, and carbon nanotubes [22-25], is of interest.
Nanomaterials, because of their unique properties, have been extensively developed, since they can act as conduction centers, facilitating the transfer of electrons and providing great catalytic surface areas [26-31].
In current years, multifunctional nanomaterials became a rich source for researchers in the field of nanotechnology, due to their enormous and wide application in the area of biomedical analysis [32] and sensing [33]. Among various types of multifunctional nanomaterials, core@shell nano-particles are the most popular, because of their dual characteristics. These particles consist of a core (the central component) and a shell (the outer component); they not only retain the characteristics of each component, but also possess unique advantages with enhanced properties [33].
In addition to the improved material properties, core@shell materials are also important from the economic point of view, e.g., one can coat an inexpensive material on lavish core material to shield it against harsh conditions or to improve its properties [34].
The technological importance of nanoparticles has driven efforts to fabricate novel core/shell nanomaterials, which are of fundamental interest to modern materials science, due to their vast applications in controlled drug release, magnetic drug targeting, inhibition of microbial biofilm growth, biosensors, antimicrobial therapy or medical diagnosis [35].
In the present work, magnetic core-shell manganese ferrite nanoparticles (MCSNP) [36] were synthesized, to modify screen printed carbon electrodes. To the best of our knowledge, so far no study has been reported on the determination of gliclazide by using MCSNP/SPCE.
Experimental
Apparatus and chemicals
The electrochemical measurements were performed by using an Autolab potentiostat/galvanostat (PGSTAT 302N, Eco Chemie, the Netherlands). The pH values were measured using a pH-meter (Metrohm 692 model, Herisau, Switzerland).
Gliclazide and all chemicals had analytical purity (Merck, Darmstadt, Germany). The phosphate buffer solution (PBS) was produced from concentrate phosphoric acid and its salts. As previously published, we synthesized the magnetic core-shell manganese ferrite NPs in our lab [36].
Preparation of the electrode
The bare screen-printed electrode was coated with MCSNP, as follows. A stock solution of MCSNP in 1 mL aqueous solution was prepared by dispersing 1 mg of MCSNP with ultrasonication for 1 h. Then, 2 µL of the MCSNP/H2O suspension solution were casted on the carbon working electrode, until the solvent was evaporated at the room temperature.
Preparation of real samples
Ten tablets of GLZ (labeled 80.0 mg per each tablet) were completely grounded and homogenized, accurately weighed and dissolved with ultrasonication in 20 mL of water. Finally, the mixture was filtered and the clear filtrate was transferred into a 100 mL volumetric flask and diluted to the mark using 0.1 M PBS with pH 7.0. Finally, a suitable volume of the resultant solution was transferred to the electrochemical cell, and the resulting solution was used for GLZ analysis. The sample was spiked with different amounts of GLZ, and the content was analyzed by using the standard addition method, in order to prevent any matrix effect.
Urine samples, immediately after collection, were stored in a refrigerator. Twenty milliliters of the sample were centrifuged for 10 min, at 3000 rpm. The supernatant was filtered using a 0.45 µm filter. In order to prevent any matrix effect, the standard addition method was used for GLZ analysis in the urine. For this purpose, a suitable volume of the solution was transferred into some 50 mL volumetric flasks, spiked with different amounts of GLZ and diluted to the mark with PBS (pH 7.0).
Results and discussion
Electrochemical behavior of gliclazide at the MCSNP/SPCE surface
The pH value of the aqueous solution is effective on GLZ electrochemical behaviour. Therefore, the solution pH was optimized to obtain highly accurate results for GLZ electro-oxidation. Accordingly, GLZ electrochemical behaviour at the MCSNP/SPCE surface was studied by voltammetry, in 0.1 M PBS, at pH values from 3.0 to 9.0. It was revealed that neither acidic nor basic media were appropriate for GLZ electro-oxidation at the MCSNP/SPCE surface, and the best results were obtained at neutral conditions (Fig. 1). Thus, the pH 7.0 was chosen as optimum for GLZ electro-oxidation at the MCSNP/SPCE surface.
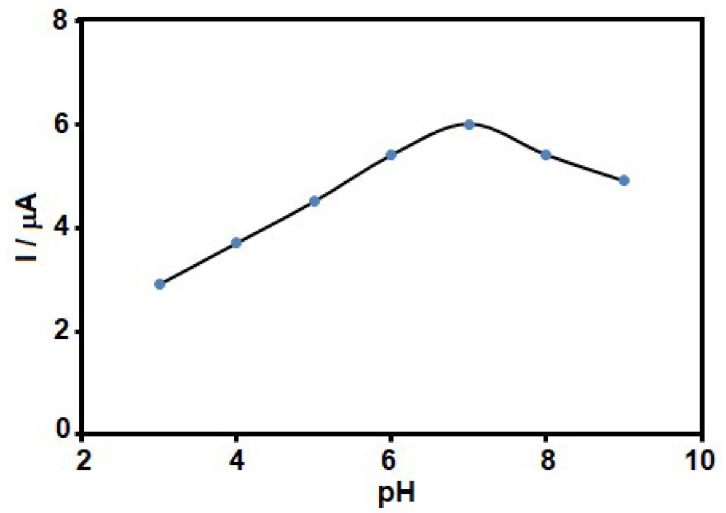
Figure 1 GLZ electro-oxidation current at the MCSNP/SPCE surface, in the presence of 80.0 µM GLZ, at various buffered pHs (3-9).
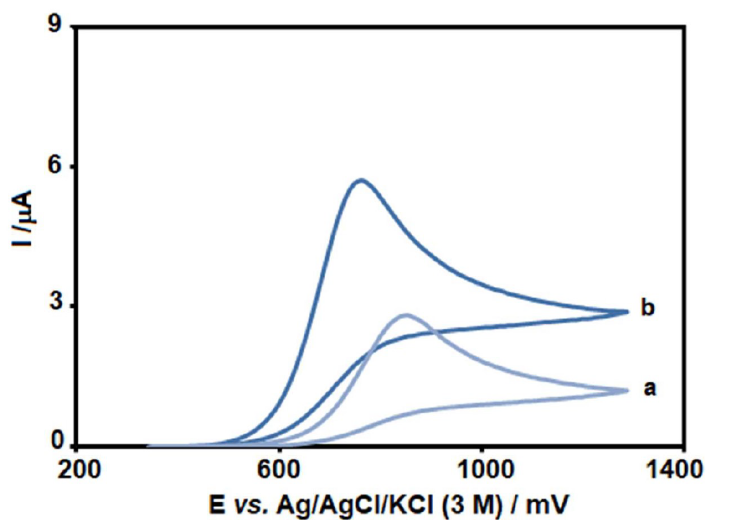
Figure 2 Voltammograms of (a) unmodified SPCE and (b) MCSNP/SPCE, in the presence of 80.0 µM GLZ, at pH 7 (at 50 mV/s).
Fig. 2 depicts the CV responses for 80.0 µM GLZ electro-oxidation at unmodified SPCE (curve a) and MCSNP/SPCE (curve b) surfaces. The peak potential, due to GLZ oxidation, occurred at 760 mV, which is about 90 mV more negative than that of the unmodified SPCE.
Also, the anodic peak current for GLZ oxidation at the MCSNP/SPCE surface is much higher, in comparison with that of the unmodified SPCE, thus indicating effectiveness of SPCE modification with MCSNP, in the GLZ oxidation process.
Effect of scan rate
LSV was used to evaluate the scan rate effect on GLZ electrocatalytic oxidation at the MCSNP/SPCE surface (Fig. 3). The results indicated that, with an increase in the scan rate, the oxidation peak potential shifted towards higher positive potentials. This shows the kinetic restriction in the electrochemical reaction. In addition, a linear plot of peak current (Ip), versus the square root of scan rate (ν1/2), was observed at the scan rate values from 5 to 1000 mV/s. This means that the process is diffusion rather than surface control at sufficient overpotential [37].
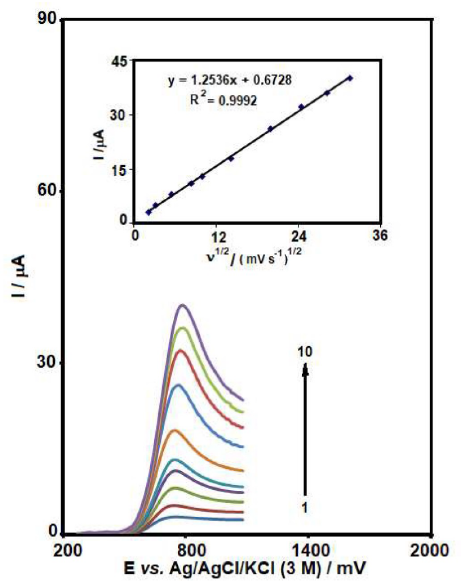
Figure 3 LSVs of MCSNP/SPCE in 0.1 M PBS (pH 7.0), containing 40.0 µM GLZ, at various scan rates; numbers 1 - 10 correspond to 5, 10, 30, 70, 100, 200, 400, 600, 800 and 1000 mV/s, respectively. Inset: variation of anodic peak current vs. square root of scan rate.
Chronoamperometric measurements
For GLZ chronoamperometry study at the MCSNP/SPCE surface, the working electrode potential was set at 0.95 V versus Ag/AgCl/KCl (3.0 M), at different GLZ concentrations, in 0.1 M PBS (pH 7.0) (Fig. 4). According to the Cottrell equation, the current of the electrochemical reaction, which is an electroactive material at the mass transport limited condition, can be seen in equation (1) [37].
I =nFAD1/2Cbπ-1/2t-1/2 (1)
where I is current (A), n is the number of electrons, F is Faraday constant (96485 C/mol), A is the electrode area (cm2), D is the diffusion coefficient (cm2/s), t is time (sec) and Cb is the bulk concentration (mol/cm3). According to the Cottrell equation, at different GLZ concentrations, diagrams of I vs. t-1/2 were drew (Fig. 4a). Then, the slopes of the obtained lines were plotted vs. GLZ concentration (Fig. 4b). From the obtained slope and from the Cottrell equation, the mean value of D for GLZ was determined as 1.1×10-5 cm2/s.
Calibration plots and limits of detection
The linearity of the peak current of GLZ oxidation on the MCSNP/SPCE surface was assessed using SWV experiment (initial potential=0.47 V, end potential=0.95 V, step potential=0.002 V, modulation amplitude=0.01995 V), in 0.1 M PBS (pH=7.0), with different GLZ concentrations.
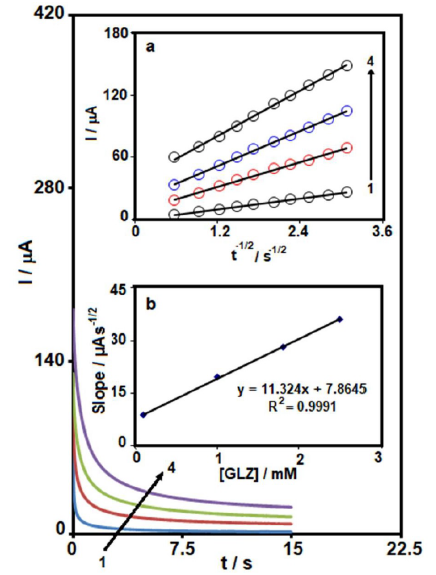
Figure 4 Chronoamperograms obtained at MCSNP/SPCE, in 0.1 M PBS (pH 7.0), with different GLZ concentrations. The numbers 1 - 4 correspond to 0.1, 1, 1.8 and 2.5 mM of GLZ. Insets: (a) Plots of I vs. t-1/2 obtained from chronoamperograms 1 - 4; and (b) Plot of the slope of the straight lines against GLZ concentration.
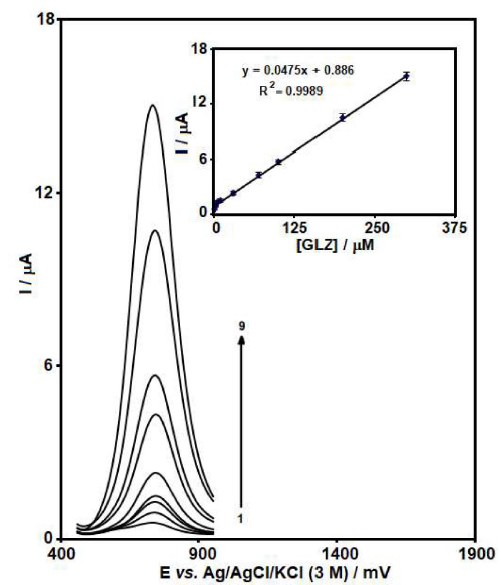
Figure 5 SWVs of MCSNP/SPCE, in 0.1 M PBS (pH 7.0), containing different GLZ concentrations (0.5, 2.5, 5.0, 10.0, 30.0, 70.0, 100.0, 200.0 and 300.0 μM). Inset: the plot of the peak current as a function of GLZ concentration is in the range from 0.5 to 300.0 μM.
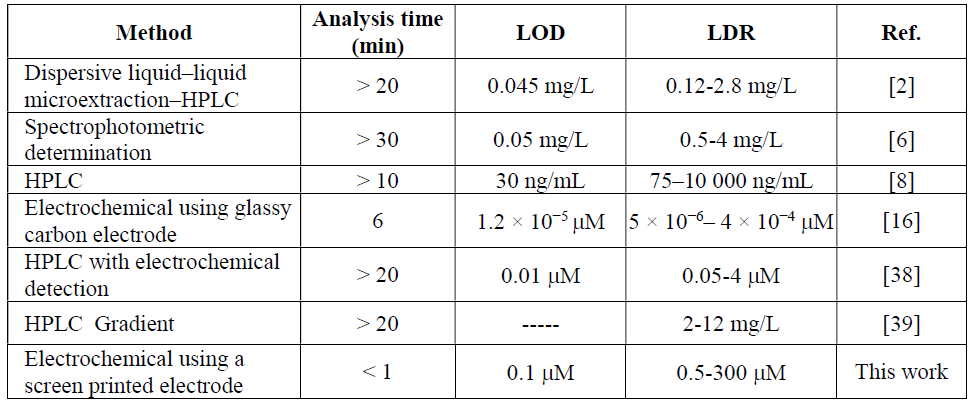
The results shown in Fig. 5 reveal that, at the MCSNP/SPCE surface, there is a linear dependency between GLZ oxidation electrocatalytic peak current and its concentration within the range from 5.0 × 10-7 to 3.0 × 10-4 M. The detected limit, on the basis of three times the standard deviation (3σ) of the blank, was 1.0 × 10-7 M. Based on our knowledge, there is only one electrochemical work on GLZ determination, and most of these methods are HPLC and GC. Therefore, our obtained results are compared with some of them (see Table 1).
Real sample analysis
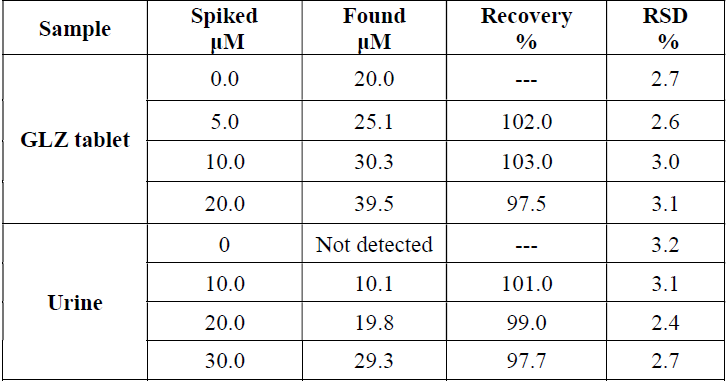
Finally, MCSNP/SPCE was applied for GLZ determination in a GLZ tablet and in urine samples. For this purpose, GLZ determination in the real samples was carried out) Table 2(.
Also, the recovery of known amounts of GLZ from spiked samples was assessed. The results show the added GLZ that was quantitatively recovered from the real sample, and demonstrate MCSNP/SPCE applicability for GLZ determination in the real samples. Also, RSD mean indicates that the method is reproducible.
The amount of GLZ in the tablet was found to be 79.63 mg/tablet. It was discovered that there is no significant difference between MCSNP/SPCE results and the nominal value on the tablet label (80.0 mg/tablet). The t-test was applied to the result, and showed that there was no significant difference, at 95% confidence level.
Conclusion
In this work, by employing magnetic core shell nanoparticles for SPCEs modification, a novel sensor that provides a sensitive method for GLZ determination has been developed. The proposed protocol demonstrated herein is a novel, simple, portable, inexpensive and easy-to-use fabrication method, for the determination of GLZ concentration in tablets and urine samples, with good analytical performance. Due to the unique properties of magnetic core shell nanoparticles, the sensor exhibited a remarkable electrochemical activity towards GLZ oxidation. Under optimized conditions, SWV exhibited linear dynamic ranges from 0.5 to 300 µM, with a detection limit of 100.0 nM.