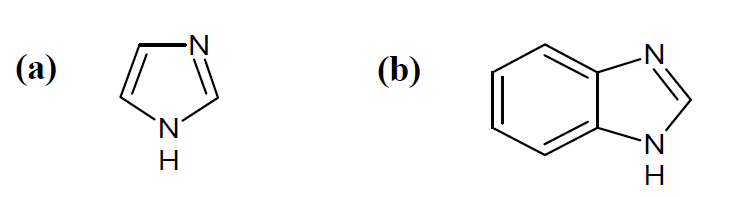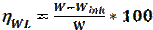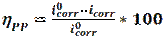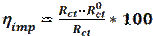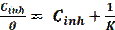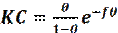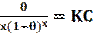Introduction
Metal surfaces are used in an extensive range of industrial applications, including petroleum, textile and food processing industries, as a component of pumps or valves that carry various types of substances [1-3]. Acid solutions are extensively used in diverse industrial fields, such as cleaning, pickling, descaling and oil-well acidizing, especially, sulphuric, hydrochloric, nitric and phosphoric acids [4]. The corrosion of metallic materials causes huge financial losses to industry, which, then, justifies the search for substances that can slow down or prevent metals corrosion rate. Nowadays, it is important to control metal corrosion, in order to expand the life of metallic equipment [5]. Corrosion is an electrochemical or chemical degradation of metals/alloys properties, due to interfacial interactions with their environment [6-7]. This process involves the displacement of metal ions into the electrolyte, at the anode. The cathodic reaction requires oxygen or hydrogen ions as “electron acceptors” [8]. The use of inhibitors is considered one of the best methods of protecting materials against the corrosion process, and it is becoming increasingly common [9-14]. The use of organic compounds as inhibitors has gained the highest priority, because they are relatively economic, effective and can be derived from commercially available starting chemicals [15]. Generally, these compounds interact through their (- and non-bonding electrons with metal, forming a surface protective film, via coordination bonds. The passive film that is formed acts as a barrier between the environment and the metal surface, thereby helping in corrosion protection [16]. The inhibiting capacity of a large number of organic compounds has been studied. Most organic compounds contain oxygen (O), sulphur (S), nitrogen (N), phosphorus (P), and multiple bonds showed significant inhibition efficiency. Recently, imidazole derivatives were reported, in different studies, as a new class of metals corrosion inhibitors, due to the availability of their π-electrons of -C=N azomethine double bond, which can coordinate with metals, to form a barrier between them and corrosive elements. The aim of this paper is to review and discuss some research works reported on corrosion inhibition, using imidazole derivatives. In addition, the main techniques for corrosion prevention reported in the literature are herein summarized.
Imidazole brief overview
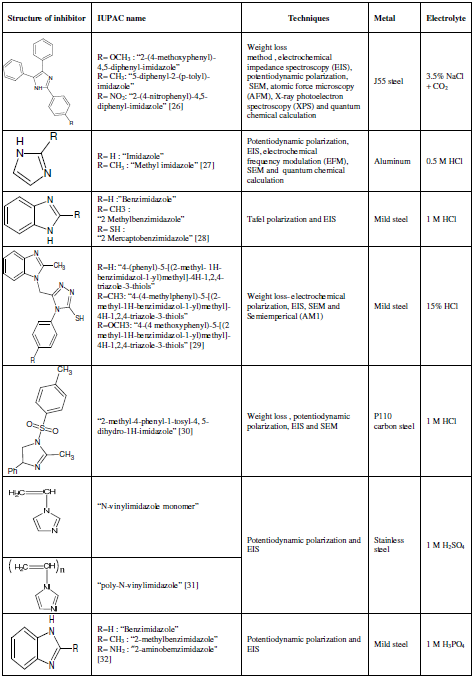
Imidazole (a) ring is a heterocyclic aromatic compound that contains three carbon and two nitrogen atoms, at 1st and 3rd positions. The systematic name for this compound is “1,3-diazole” [17]. Benzimidazole (b) has a benzene ring fused in the 4th and 5th position.. The molecular structure of (a) and (b) is presented in Fig.1.
The imidazole ring system is, of course, not just interesting and a source of endless research pleasure, but it is also a key system, in its compounds (such as the amino acid “histidine”, vitamin B12, a component of DNA base structure, purines, histamine, biotin, etc.); thus, it is an important constituent of many natural products, in pharmaceutical, veterinary and agrochemical products [18]. It also has alkaloids that possess several biological activities, such as antibacterial, anticancer, antitubercular, antifungal, anti HIV, anti-protozoa, anti-inflammatory, anti-mycobacterial, anxiolytic and anti-diabetic activities [19-20].
Imidazole derivatives as corrosion inhibitors in aqueous media
Recently, imidazole has attracted a lot of attention in the field of metallic corrosion inhibition, due to its interesting properties, low cost and ease of synthesis [21]. It is also environmental friendly, exhibiting significant inhibition efficiency. Imidazole derivatives have been evaluated, by several investigators, as effective corrosion inhibitors, in various media. For example, Yanardag et al. reported the corrosion inhibition efficiencies of some benzimidazole derivatives, for zinc, brass and copper, in alkaline and neutral media [22]. Xinkuai He et al. [23], Ingrid Milosev et al. [24] and M. Mahdavian et al. [25] have also reported that imidazole derivatives are effective organic corrosion inhibitors on aluminium, copper and steel. This paper investigates the inhibition potentials of several imidazole derivatives exposed to different media. Table 1 displays the chemical structures of eight imidazole derivatives used in this review, in order to study the inhibitive property of these compounds on various metals (mild/stainless/J55/ P110 steel and aluminum) in different media (HCl, NaCl+CO2, H2SO4 and H3PO4).
Technical details and definitions
Weight loss
Weight loss is the most commonly and the most accurate method used to determine corrosion rate in several studies [33-35]. Often, this technique does not require expensive equipment. The specimens are immersed in corrosive media, for a specific period. Weights changes of the specimens, before and after immersion in corrosive environments, were measured by using an electronic balance. The inhibition efficiency (ηWL) can be estimated by using the following equation [36]:
where W and Winh are the values of the corrosion rate, in the inhibitor absence and presence, respectively.
Potentiodynamic polarization measurements
Potentiodynamic polarization is used to determine the data of corrosion potential (Ecorr) and current density (icorr) [37]. The system consists of a three electrode unit directly connected to a potentiostat. The three electrodes are the working electrode (WE), reference electrode (RE), and counter (or auxiliary) electrode (CE). The reference electrode most commonly used is the saturated calomel electrode (SCE) [27-30, 38, 39] and Ag/AgCl [25, 31]. For an electrochemical reaction under pure activation control, polarization curves exhibit a linear behavior in the applied potential vs. current or log (i) plots, called Tafel behavior (i.e., linear on semi-logarithmic scale) [37]. Corrosion rate or corrosion current density (icorr) is obtained by extrapolating both cathodic and anodic Tafel regions back to the corrosion potential (Ecorr). The corrosion inhibition efficiency (ηpp) is determined by following equation [40-42]:
where 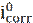 and
and 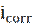 are the corrosion current densities values, in the inhibitor absence and presence, respectively.
are the corrosion current densities values, in the inhibitor absence and presence, respectively.
Electrochemical impedance spectroscopy (EIS)
Electrochemical impedance spectroscopy (EIS) is a powerful and effective technique which can be used to study the electrical properties of the electrode/ electrolyte interface. EIS is used to characterize electrochemical systems and measure impedance changes in these systems [43]. The principle of this technique is to apply a small amplitude (1-10 mV) ac sinusoidal signal of potential or current, in a wide range of frequencies, to an electrode inserted into an electrolyte, in order to measure the current response [43]. The EIS measurements are interpreted through graphical representations. The Nyquist plot represents the impedance imaginary part vs the impedance real part (Z = Zreal + jZim). The second representation is called Bode diagram, which represents the logarithm of the impedance modulus (log|Z|) and the phase shift vs. the frequency logarithm (log f) [44]. The inhibition efficiency (ηimp) was determined using the following equation [45-46]:
where 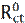 and Rct are the charge transfer resistance values, in the inhibitor absence and presence, respectively. The interpretation of the impedance spectra requires the selection of an “equivalent circuit” that suitably fits the experimental data [47-49]. Nyquist plots, which possess incomplete capacitive semicircles, characterize a non-ideal capacitance behavior, leading to the inclusion of a constant phase element (CPE) in the proposed equivalent circuit. G. Karthik and M. Sundara Vadivelu [50] have introduced this element into the equivalent circuit, to fit the impedance data.
and Rct are the charge transfer resistance values, in the inhibitor absence and presence, respectively. The interpretation of the impedance spectra requires the selection of an “equivalent circuit” that suitably fits the experimental data [47-49]. Nyquist plots, which possess incomplete capacitive semicircles, characterize a non-ideal capacitance behavior, leading to the inclusion of a constant phase element (CPE) in the proposed equivalent circuit. G. Karthik and M. Sundara Vadivelu [50] have introduced this element into the equivalent circuit, to fit the impedance data.
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy (XPS) is a quantitative technique that is capable of identifying elemental compositions and analyzing the outermost molecular layers of a material [51]. XPS is a surface characterization technique based on the photoelectron effect, i.e., emission of an electron following the excitation of core level electrons by photons. This technique surveys the electron binding energy spectrum of a sample surface, resulting in a plot of binding energy versus total electron count [52].
Scanning electron microscopic (SEM)
SEM is the best known and mostly applied of the surface analytical methods [53]. SEM is able to obtain information, such as the texture (morphological aspects) and orientation of components, as well as the crystalline structure of the sample. Different models are used to characterized the surface morphology in some works that have been reported in this article, such as: JOEL, JSM-T20, Japan [27]; JEOL JSM - 6380, LA [29]; and SEM JSM-7500F, Japan [30].
Atomic force microscopy (AFM)
The AFM technique is a powerful tool used to quantify the surface morphology of a metal/solution interface [54]. This technique was developed from scanning tunnel microscopy with nanometer resolution [55]. Atomic force microscopy is a high precision technique that provides the material topography and chemical composition of a metal surface, with high spatial resolution [54]. Some authors in the field of corrosion have employed AFM , for example, Yujie Qiang et al. (2017) and Paulina Arellanes-Lozada et al. (2018) [56, 57].
Quantum chemical calculations
Theoretical chemistry, such as quantum chemical calculations, has been recently used to analyze the adsorption mechanism of inhibitors on a metal surface. The use of quantum chemical calculations is very important, in order to establish correlations between the inhibitive effect and the structure of the molecule [58]. Recently, computational modeling techniques have been successfully applied to corrosion phenomena, as summarized in some articles by J. Haque et al. [59] and N. Anusuya [60]. A. Singh et al. have studied the corrosion inhibiting effect using density functional theory (DFT) method, with hybrid function of Becke three parameters; the same was made by Lee, Yang and Parr (B3LYP), with 6-31G (d) basis set. L. H. Madkour et al. and I. H. Elshamy [61] have also used DFT/B3LYP with 6-31G (d,p), for seven benzimidazole derivatives. The molecular geometry has a significant influence on the inhibitor adsorption onto a metal surface, as it informs of the optimal way by which the inhibitor might cover the metal surface [1]. Inhibitor molecules that have high electron planar geometry always produce stronger adsorption onto the metal surface than those molecules that have less planar geometry [62]. Quantum chemical parameters, such as EHOMO, ELUMO, energy gap (∆Egap), dipole moment (µ), total energy (TE), absolute hardness (η) and absolute electronegativity can be estimated, in order to study the chemical reactivity of molecules and their inhibition potentials.
Adsorption of corrosion inhibitor and its isotherms
Organic corrosion inhibitors reduce the corrosive attack through the adsorption onto the material surface, followed by the formation of a protective layer [63]. According to Moretti [64], organic inhibitors can be adsorbed onto the interface metal/solution, by replacing one or more adsorbed water molecules (H2Oads) in the aqueous solution, as shown by the following equation:
Org(sol) + n H2O(ads) ( Org(ads) + n H2O(sol) (4)
where n is the number of bipolar molecules of water replaced by one inhibitor molecule. The adsorption process depends on the metal surface charge, type of electrolyte, molecular structure and electrical characteristics of an organic inhibitor [65]. The adsorption process of corrosion inhibitors onto the metallic surface can be described by two main types of interaction: physisorption and chemisorption. The first type is the result of electrostatic and Van Der Waals interactions between the organic ions and the electrically charged metal surface [66]. De Philip A. has also reported that chemisorption involves charge transfer from the inhibitor molecules to the atoms of the metal surface, in order to form a coordinate type of bond [66]. The interactive nature of corrosion inhibitors with metal surfaces can be explained using various adsorption isotherm models.
Langmuir isotherm
The Langmuir isotherm model explains adsorption by assuming monolayer coverage on a homogeneous surface with identical adsorption sites [67]. Langmuir isotherm, which represents the best fit of experimental data, can be expressed as follows [67]:
where ( is the degree of surface coverage, K is the constant describing the adsorption/desorption equilibrium and C is the inhibitor concentration.
Frumkin isotherm
The adsorption process of corrosion inhibitor described by the Frumkin isotherm is given by the following equation [68]:
where ( is the degree of surface coverage, C is the inhibitor concentration, f is the constant of attraction and K is the equilibrium constant of adsorption/desorption. The value of f parameter depends on the interaction between an adsorbed molecule and a metallic surface and also on the degree of surface heterogeneity.
Flory-Huggins
The Flory-Huggins model is believed to be a substitutional model, i.e., which describes the substitution of inhibitor molecules for water molecules [68]. The Flory-Huggins adsorption isotherm can be linearly expressed as follows [69]:
where θ is the degree of inhibitor surface coverage, K is the equilibrium constant of adsorption/desorption and x is the number of water molecules replaced by the inhibitor molecule. M. Saadawy [70] has used Flory-Huggins model to fit the corrosion data of three anions in the H2SO4 solution.
Results and discussions
Corrosion rate and inhibition efficiency of reported imidazole compounds (Table 1), in different media, have been studied. Percentage inhibition efficiency values, obtained through weight loss, potentiodynamique polarization and electrochemical impedance spectroscopy techniques, are given in Table 2.
Table 2 Extracted results obtained on the corrosion inhibition behavior of different compounds in different media.
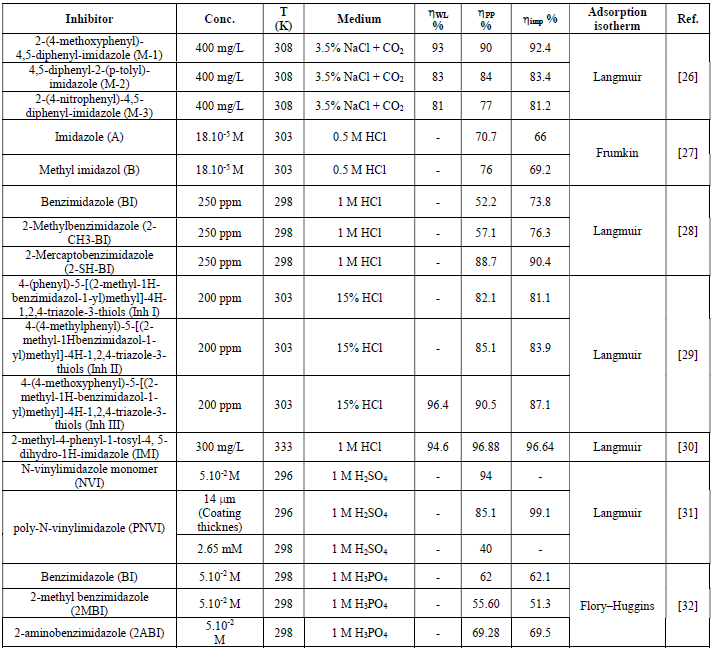
The corrosion behavior of J55 steel, in 3.5% NaCl saturated with a CO2 solution, in the absence and presence of three imidazole derivatives, was investigated by A. Singh et al. [26], using various techniques: weight loss, EIS, and potentiostatic polarization. The authors found that the inhibition action of the tested compounds depends on their concentration and chemical structure. M-1 represents the better inhibitor in the given series, exhibiting the best inhibition efficiency of 93%, at the concentration of 400 mg/L, after 3h, due to the presence of methoxy (OCH3),as an electron donating group. The adsorption process followed Langmuir isotherm. In the presence of these compounds, J55 steel surface is shown in SEM and AFM micrographs, exhibiting a smooth and uniform morphology, due to the formation of a passive film on the metal surface. Surface analysis (XPS) was also investigated, to discuss the inhibition mechanism of the studied inhibitors on J55 steel, in a 3.5% NaCl + CO2 medium.
M. N. El-Haddad et al. [27] studied the electrochemical behaviour of aluminum in a 0.5 HCl solution with imidazole (A) and methyl imidazole (B). The addition of (A) and (B) compounds led to an increase in the corrosion inhibition efficiency. The maximum corrosion inhibition efficiencies obtained by potentiodynamique polarization were 70.7% for (A) and 76% for (B), at a 18.10-5 M concentration. The corrosion inhibition process was based on the adsorption of these compounds and on the formation of an adhered layer onto the metal surface. This film was characterized using scanning electron microscopy (SEM). The nature of inhibition was found to be of mixed type, predominantly cathodic. EIS measurements were interpreted and explained according to a suitable circuit model, using fitting software. Some activated thermodynamic parameters were computed, indicating that both physical and chemical adsorption may occur on aluminum surface. Furthermore, some quantum chemical parameters, as well as some other structural parameters, have been calculated to understand the inhibition mechanism of imidazole derivatives.
The effects of Benzimidazole (BI), 2 Methylbenzimidazole (2 CH3-BI) and 2 Mercaptobenzimidazole 2-(SH-BI) on mild steel, in a 1 M HCl solution, were studied by J. Aljourani et al, using polarization and impedance measurements [28]. The corrosion current densities (icorr) decreased in the following order: BI > 2 CH3-BI > 2-SH-BI. This result indicates the high beneficial effect of 2-SH-BI ((imp = 90.4% at 250 ppm) on mild steel corrosion inhibition, in molar hydrochloric acid. Adsorption isotherm results demonstrated that the adsorption process obeyed Langmuir isotherm. Rtc has increased with higher concentrations of the tested compounds. Cdl values have decreased and were brought down to the maximum extent, with higher inhibitors concentration, which was most probably caused by the increase in the thickness of the electrical double layer. This suggests that these inhibitors act via adsorption at the metal/electrolyte interface. The obtained thermodynamic parameters of adsorption indicated that the corrosion inhibitor mechanism has retarded corrosion processes, through physical adsorption.
The inhibiting activity of some substituted imidazoles in 15% HCl, in relation to inhibitor concentrations, using electrochemical techniques, was studied by M. Yadav and S. Kumar. The inhibition efficiencies followed the order: Inh I < Inh II (-CH3) < Inh III (-OCH3). The adsorption of these compounds onto the mild steel surface obeyed the Langmuir isotherm model. The polarization curves revealed that all the studied inhibitors acted as mixed type inhibitors. The calculated values of the free energy of adsorption suggested that the adsorption of the three molecules involved chemisorption. The inhibitors created a smooth surface, indicating that the corrosion rate was significantly reduced. The geometry optimizations of the three molecules were obtained by application of the unrestricted Hartre Fock (UHF) method, using AM1. The highest value of EHOMO (-8.6541 eV) and the lowest value of ∆E (7.9472 eV) reflects a higher adsorption capability of Inh III onto the mild steel surface than that of Inh I and Inh II.
The inhibiting effect of 2-methyl-4-phenyl-1-tosyl-4 and 5- dihydro-1H-imidazole on the dissolution of P110 carbon steel, in a 1 M HCl solution, was studied by Lei Zhang [30], using weight loss measurements, EIS and potentiodynamic polarization techniques. The author, using EIS technique, concluded that the inhibition effectiveness increased with higher additive concentrations, reaching 96.64% at 300 mg/L. The adsorption isotherm confirms the applicability of Langmuir’s equation to explain the adsorption process of the tested molecule. The displacements of corrosion potential were lower than 85 mV, compared to those of the uninhibited solution, indicating that IMI acted as a mixed-type inhibitor. The SEM analysis indicates that IMI can remarkably protect carbon steel from aggressive media, due to the formation of a protective layer on the metal surface.
The inhibitive action of the extract of two imidazole derivatives, namely N-vinylimidazole monomer and poly-N-vinylimidazole, on stainless steel, in a 1 M H2SO4 acid medium, was investigated by Öncül et al. [31], using weight loss, potentiostatic and potentiodynamic polarization techniques. Experimental results clearly demonstrate the ability of either PNVI or its monomer to reduce the corrosion process that occurs on stainless steel. The inhibition efficiency increases with a higher monomer concentration. The best inhibition, 94%, was obtained by adding 5.10-2 M of monomer into the corrosive environment. The inhibition mechanism of N-vinylimidazole was discussed in view of Langmuir isotherm model. The inhibitory character of the polymer depends upon the thickness and porosity of the film. The increase in thickness and the decrease in porosity improved the coating corrosion resistance.
The inhibition performance of three imidazole compounds on mild steel corrosion, in a 1 M H3PO4 solution, was studied by A. Ghanbari [32], using electrochemical measurements. The inhibition performance depended on the chemical structure and followed the sequence 2ABI > BI > 2MBI. The best fit of the impedance data was obtained using a constant-phase element (CPE), rather than an ideal capacitor. The charge transfer resistance of Benzimidazole (BI), 2 aminobenzimidazole (2ABI) and 2-methyl Benzimidazole (2MBI) increased slightly, indicating the formation of an insulating protective film at the metal/corrosive medium interface. The obtained results show that these compounds were adsorbed onto the mild steel surface, following Flory-Huggins isotherm model, and that each inhibitor molecule replaced 3-5 molecules of water molecules on the steel surface. The inhibited solution pH moved towards more alkali levels, and the solution resistance (Rs) increased with higher concentrations of the tested compounds, which indicates protonation of inhibitor molecules. 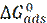 shows that the inhibitors adsorbed onto the steel surface, through physical and chemical adsorption.
shows that the inhibitors adsorbed onto the steel surface, through physical and chemical adsorption.
Conclusion
The finding of new and efficient imidazole derivatives has been the goal of many research projects. Corrosion inhibition depends on the molecular structure of the inhibitors and on their affinities for the metal surface. The most efficient corrosion inhibitors are those compounds containing hetero atoms, such as nitrogen (N) and oxygen (O), as well as aromatic rings. According to the chemical and electrochemical techniques, it is obvious that the metal corrosion rate decreased with higher inhibitor concentrations. Imidazole derivatives exhibited relatively high inhibition efficiencies for various industrial metals, in different mediums. All the inhibitors interacted with the metal surface, forming a passive film or an adsorbed layer that acted as barrier films.