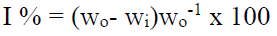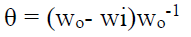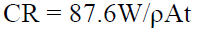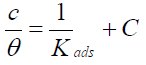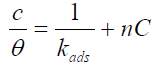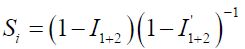Introduction
Steel of different grades is extensively used in different domestic and industrial applications. It has equally been observed that industrial acid pickling and oil-well acidizing cause rapid corrosion of steel structures (1, 2). The industry is replete with various technological attempts at proffering solutions for preventing corrosion through material selection, novel design strategies and the deployment of diverse protective techniques. The application of inhibitors as a reliable approach to the metallic materials protection from corrosion is well documented. (1-24). The use of eco-friendly and biodegradable green inhibitors, obtained from cheap and readily available naturally occurring extracts of plant and animal origin, has generated much interest in recent times. Extracts from several plants have been reported to inhibit the metals corrosion in acidic media (2, 12-24).
The present study investigated the Pinus oocarpa seed extract inhibitive effects on galvanized steel corrosion in hydrochloric acid (HCl), using the gravimetric method. The potassium iodide (KI) synergistic effect on the extract inhibition efficiency was also analysed. The obtained result is aimed at developing an efficient, biodegradable and environmentally friendly protection that will prevent material loss and reduce corrosion control economical costs. P. oocarpa is non-toxic and biodegradable. It is also readily available in the Nigerian flora.
P. oocarpa is of the family pinaceae, it is as an egg-cone pine and an evergreen tree. It has been reported as a medicinal plant (25) and it is an important commercial plant for wood production in the paper industry (26). Rubio, et al. (27) found that the P. oocarpa major constituent - oleoresin - contains two effective diterpenes on trypanosomacruzi epimastigotes. Philipson and Wright (28) have shown that terpenes extracted from this plant are active against parasitic protozoans.
Materials and methods
Galvanized steel coupons, with the dimensions of 4.5 cm by 3 cm, were polished using various emery paper grades. The polished coupons were washed with distilled water, degreased with absolute ethanol followed by acetone, dried and stored in an air-tight desiccator, to avoid moisture prior to use (10, 14).
P. oocarpa seeds were collected from the flora of the Olabisi Onabanjo University Mini-Campus, Ago- Iwoye, and classified at the Federal Research Institute of Nigeria (FRIN), FHI number 108780. The seeds were dried and ground to powder. Continuous solvent extraction was carried out with methanol. The excess solvent was distilled, and the extract was left overnight to crystallize. The crystals obtained from the powdered seed extract were stored in an air-tight desiccator and used without further purification. Inhibitor test solutions were prepared at the concentrations from 1 g to 5 g (W/V), with an appropriate quantity of 2 M HCl, in a total volume of 100 mL.
Previously prepared galvanized steel was weighed and immersed in 100 mL of different test solution concentrations. The coupons were retrieved every 2 h, washed and reweighed. The differences in the coupons weight were taken as the weight loss evaluated in grams. Corrosion investigations were undertaken at the temperatures of 303 K and 333 K, and in 2 M HCL.
The P. oocarpa inhibition efficiency was calculated using the equation:
The degree of surface coverage (θ) was calculated from the equation:
and corrosion rate (mm/yr.) was evaluated using the formula:
where, for galvanized steel, W is the weight loss (g), ρ is the density given as 7.85 x 10gcm-3, A is the cross -sectional area (cm2) and t is exposure time in h.
The halide additive synergistic effect was studied by adding 10.0 mM KI to 3 g of the P. oocarpa extract test solution.
Results and discussion
P. oocarpa extract inhibition efficiency on galvanized steel corrosion and weight loss
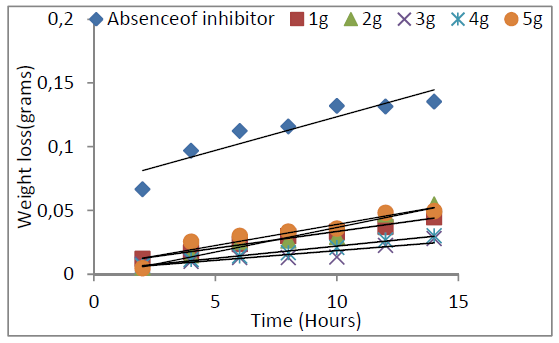
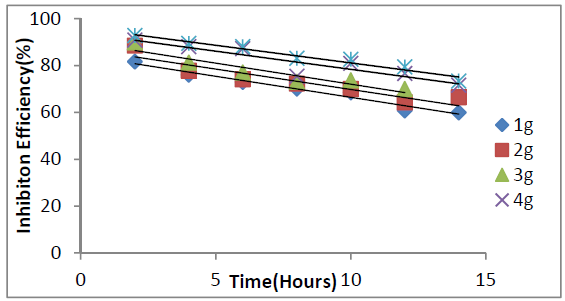
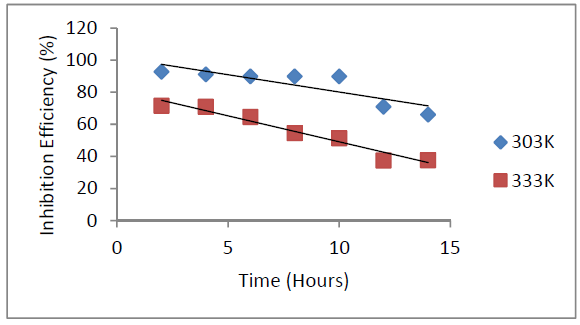
Galvanized steel weight loss decreased with time and P. oocarpa seed extracts higher concentrations (Fig. 1). The inhibitor efficiency decreased with time and increased with its higher concentrations (Fig 2). However, its inhibition efficiency decreased with a rise in temperature (Fig. 3). These observations presuppose that the inhibitor has covered a larger metal surface area, when its concentration was increased. The decrease in P. oocarpa inhibition efficiency with higher temperatures depicts the inhibitor physical adsorption mechanism onto the metal surface. Fig. 4 shows the corrosion rate temperature dependence on the inhibitor concentration.
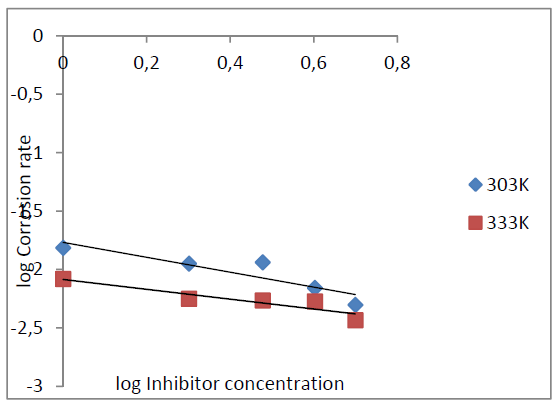
The corrosion rate increases with a rise in temperature and decreases with higher inhibitor concentrations (Fig. 4).
Thermodynamic parameters of galvanized steel corrosion in 2 M HCl
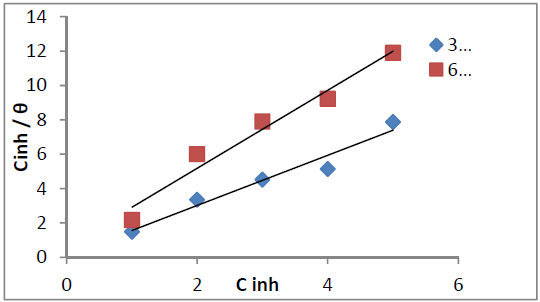
The P. oocarpa surface coverage values best fitted to Langmuir model (Fig. 5) represented by the equation (4):
The P. oocarpa seed extract composition is complex. Therefore, its components may be attractive or repulsive towards the galvanized steel surface, and its adsorption may occur at the anodic or cathodic sites of the latter. Thus, the adsorption onto the galvanized steel surface is represented by the modified Langmuir isotherm shown as equation (5):
where C is the inhibitor concentration, θ is its surface coverage area and n is a measure of its inhibition efficiency.
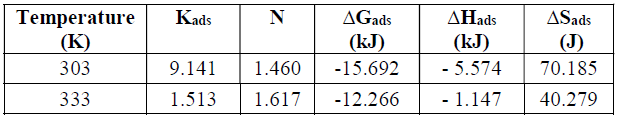
Table 1 shows the thermodynamic parameter obtained from the Langmuir’s adsorption isotherm. The obtained free energy of adsorption is negative, and lower than -20 kJ/mol. The equilibrium constant of adsorption, Kads, decreases at higher temperatures. The inhibitor efficiency (N) was enhanced as the temperature was increased from 303 K to 333 K. These observations suggest that P. oocarpa was physically adsorbed by the metal. The enthalpy of adsorption, ∆Hads, reported for this study was obtained from the Van’t Hoff’s equation, it is negative, and its magnitude increases at higher temperatures. So, this observation implies that the inhibition efficiency decreases as the temperature increases.
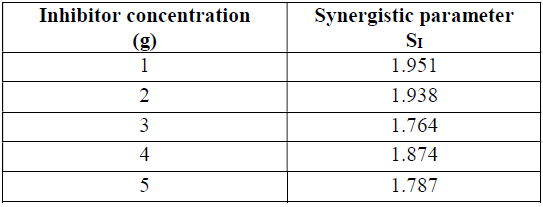
Iodide ions synergistic effect on P. oocarpa extract inhibitive properties
The synergistic parameter obtained in Table 2 has been calculated from the Aramaki and Hackerman equation (29).
where I1+2 = I1 is the halide ion inhibition efficiency, I1 is the P. oocarpa extract inhibition efficiency and Iʹ is the halide and P. oocarpa extract measured inhibition efficiency.
The synergistic parameter values obtained in this study are greater than 1, which suggests a synergism between iodide ions and the P. oocarpa seed extract. It has been reported that the iodide ions strong adsorption and the electrostatic interactions of the inhibitor cations with the metal surface enhance inhibition efficiency (19, 30-31). Thus, the chemically adsorbed P. oocarpa iodide ions effect, on galvanized steel, increased the inhibitor adsorption surface area and, thereby, enhanced the extract inhibition efficiency.
Fig. 6 shows that the iodide ions synergistic effect is more pronounced at 60 ºC, when compared to that at the lower temperature of 30 ºC.
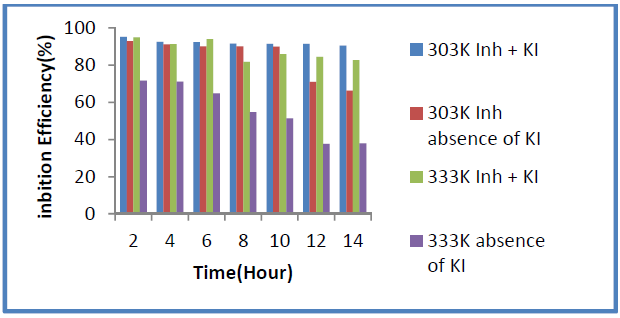
Figure 6 Temperature dependence of the 10 mM KI synergistic effect on P. oocarpa inhibition efficiency.
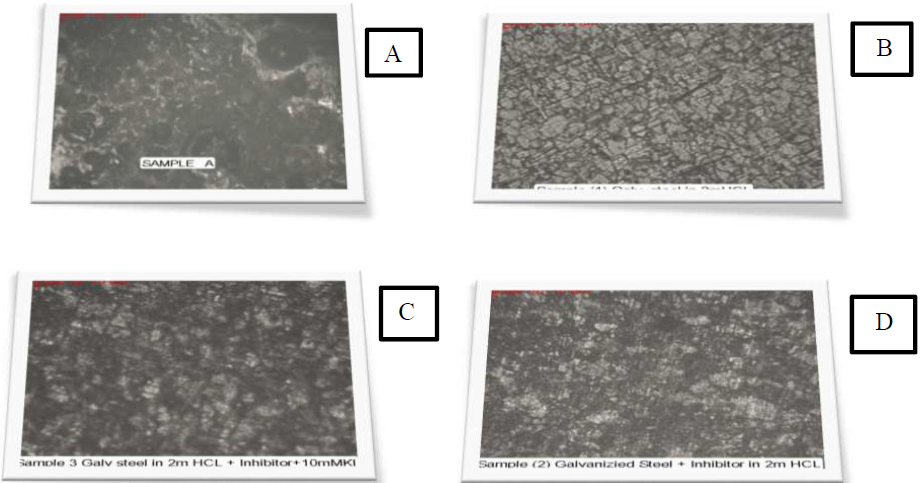
Galvanized steel optical microscopy with P. oocarpa extract
Fig. 7(A-C) shows the optical micrographs obtained for this study using the Foundrax Brinell Microscope type 2BM15, serial number 100332. Fig. 7A and 7B shows galvanized steel before and after immersion in a corrosive environment (2 M HCl), respectively. The metal surface in Fig. 7A is smooth, with minimum cracks that are greater in Fig 7B. The cracks observed in Fig. 7B may resulted from the combination of applied stress and the acidic solution (32). Fig. 7C is the micrograph of galvanized steel immersed in 2 M HCl with P. oocarpa. Fig. 7D is the micrograph of galvanized steel to which the inhibitor and 10 mM KI were added. The cracks observed in Fig. 7B appeared to have been significantly filled, and in Fig. 7C are even less pronounced, due to the synergism between KI and the inhibitor, as shown in Fig. 7D. This implies that P. oocarpa is an effective inhibitor and can be used as a surface coating for galvanized steel.
Conclusion
P. oocarpa seed extract acts as an efficient inhibitor of galvanized steel corrosion. The extract inhibition efficiency increased with its higher concentration and decreased with the longer immersion time of the metal. However, the inhibitor efficiency decreased at higher temperatures, which presupposes a physical adsorption mechanism. P. oocarpa inhibitive effect was enhanced by the potassium iodide addition to the test solution. The inhibitor reduced the inter-granular stress cracks in the galvanized steel used for this study.
Acknowledgement
The author is grateful to the Midland Galvanizing Company, Abeokuta, especially, to Mr Adams, for having supplied galvanized steel for this study, and to Mr Habib, of Nigeria Foundry Limited, for having carried out the optical microscopy studies using the Foundrax Brinell Microscope type 2BM15, serial number 100332.