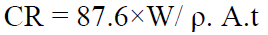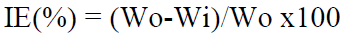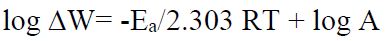Introduction
Drinking water is commonly transported through cast-iron pipes, but metal corrosion causes a lot of trouble in the water delivery pipeline system [1-2]. Iron pipes undergo oxidation, producing iron oxide (Fe2O3H2O), known as scale. The scale then accumulates inside the pipeline and forms tubercles which adversely affect the water flow through the pipes and usually cause public complaints about “red water” at the tap. All these problems lead to water pollution in the distribution system [3]. Nowadays, many methods are available to minimize this type of corrosion in water industry. Many of the conventional inhibitors possess high toxic nature, and they may cause health problems in humans and animals. Therefore, recently extensive efforts have been made to develop green, smart, safe and multifunctional corrosion inhibitors. Some plants are proved efficient corrosion inhibitors of metal substrates in drinking water [4-6]. Our aim is to focus on the selection of an environment friendly new inhibitor for preventing mild steel corrosion products in drinking water. Pimenta Dioica, commonly known as allspice, has wide applications in the medical and non-medical fields [7-8]. Although properties, such as antimicrobial, insecticidal, antioxidant, antidiabetic and anticancer activities, have been investigated [9], its ability to prevent corrosion has not been reported. This research work investigates the allspice leaf powder inhibition of mild steel corrosion in potable water, and the various steps involved in the process are discussed below.
Experimental part
Substrate preparation
Mild steel coupons containing 0.4% carbon, 0.2% manganese, 0.024% phosphorous, 0.1% silicon, 0.05% sulphur and 99.226% iron, were used as substrates for the study. The selected mild steel coupons dimensions were 5 x 4 x 0.5 cm. Different grades of emery paper (100, 200, 400, 600 and 800) were used to polish the substrate surface. Each emery paper was used to polish the metal sample surface successively for five min. After mechanical treatment, the plates were cleaned using distilled water, dried using acetone and kept in desiccators until use. P. Dioica leaves that were used for this study were obtained from Sasthamcotta, Kollam district. The leaves were thoroughly washed with distilled water, dried by sunlight and cut into small pieces, and then crushed into a fine powder. Powdered leaves were then subjected to the study of its corrosive prevention effect in suitable media.
Weight loss measurements
Mechanically cleaned plates were subjected to weight loss measurements, immersed at room temperature in 100 mL tap water taken in a beaker, without and with the inhibitor, at different concentrations (0 g/100 mL, 1 g/100 mL, 2 g/100 mL, 3 g/100 mL, 4 g/100 mL and 5 g/100 mL). After 2 h, the coupons were thoroughly cleaned using deionized water, well dried and weighed using a Schimadzu AUX220 balance. After weighing, the plates corrosion rate (CR) and the P. Dioica inhibition efficiency (IE) were determined using the formula,
where W is the weight loss in mg, ρ is density (7.85 g/cm3 for mild steel), A is the area in cm2 and t is exposure time in h. The experiments were conducted using coupons with a surface area of 4 cm2, and the remaining surface was covered using Teflon.
where Wo and Wi are the weight loss value in the inhibitor absence and presence, respectively.
OCP variation with time
The open circuit potential is the electrode equilibrium electrode potential, and it determines the electrode corrosion tendency. The plates OCP, in water media with different inhibitor concentrations, was measured with reference to the calomel electrode, using a digital multimeter. The inhibitor concentrations selected for the present studies were 0%, 1%, 2%, 3%, 4% and 5%, respectively.
Results and discussion
Weight loss studies
Fig. 1 and Fig. 2 represent the plates CR and the P. Dioica IE, respectively, in tap water. From the figures, it is clear that, with up to 3% of the inhibitor concentration, the corrosion rate decreases. After that, with higher allspice leaf powder concentrations, the corrosion rate slowly increases. That is, at higher concentrations, the inhibitor molecules accelerate corrosion. This happens when the inhibitor favors mild steel dissolution in water. When an inhibitor concentration of 3 g/100 mL was added, the metal corrosion rate was found to be 3.5 x 10-4 mpy, and the corresponding inhibition efficiency (IE) was 77%. As above discussed, the addition of higher inhibitors concentrations (4 g and 5 g) caused a decrease in inhibition efficiency. At higher concentrations, the adsorbed layer formed on the metal surface may dissolve and go into the solution. This type of reaction accelerates the substrate corrosion rate.
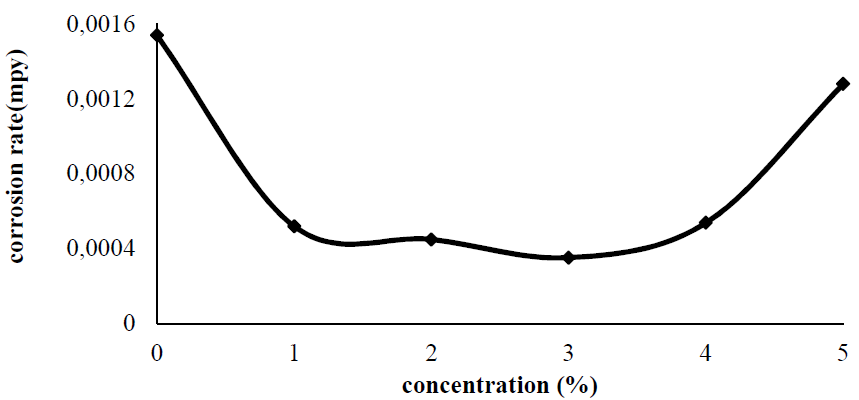
Figure 1 Mild steel coupons corrosion rate variation with different P. Dioica leaf powder concentrations in tap water, at 32 ºC.
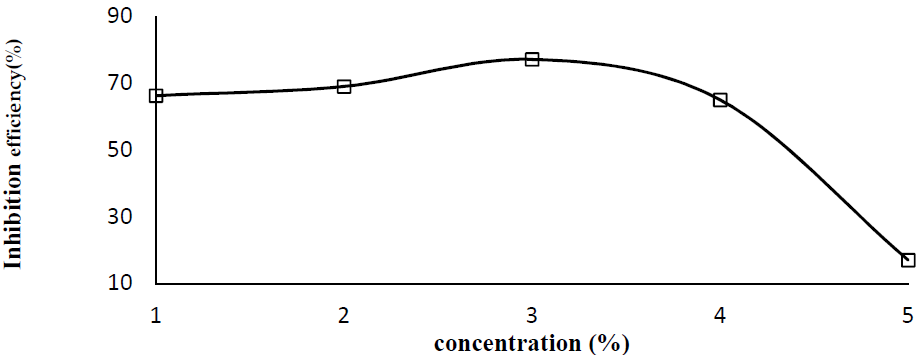
Figure 2 P. Dioica leaf powder inhibition efficiency variation with different concentrations, for mild steel coupons in potable water, at 32 ºC.
Electrochemical evaluation
An open circuit potential study was conducted with different P. Dioica leaves powder concentrations (0%, 1%, 2%, 3%, 4% and 5%) in potable water, and its variation with time is shown in Fig. 3. Here, we find that, at the time of coupons immersion in the water with inhibitor, the corrosion potential varied from -657 to -717 mV vs. SCE.
The coupon in potable water without inhibitor exhibited a potential of -567 mV. Coupons immersed in the corrosive media, with different inhibitor concentrations, exhibited an anodic behavior after 22 h of exposure.
This positive potential shift indicates the formation of an oxide passive film on the substrate surface [8, 11]. The coupon without inhibitor in tap water showed only cathodic behavior throughout the experiment.
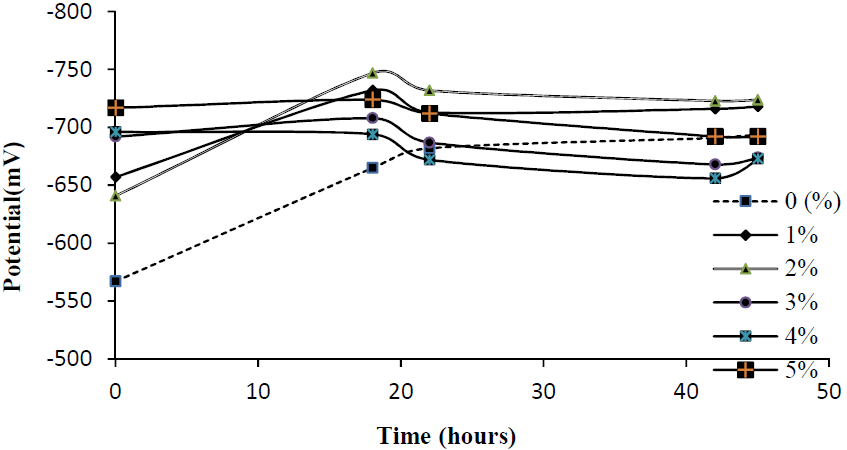
Figure 3 OCP variation with time for a mild steel electrode immersed in tap water, in the absence and presence of different P. Dioica leaves powder concentrations.
Temperature effect
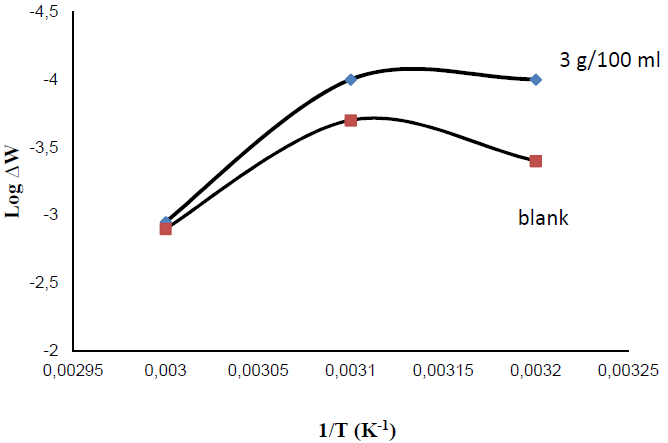
In the present study, a temperature range from 300 K to 330 K was selected for studying its effect on the corrosion rate, for half an hour, in tap water with and without inhibitor. Optimum inhibitor concentration (3 g/100 mL) was chosen for this study. From Fig. 4, it can be inferred that the substrate corrosion rate in the solution with inhibitor remains almost the same up to 320 K. After that, the corrosion rate increases with a rise in temperature. High temperatures in the system cause the inhibitor molecules to desorb from the substrate surface. According to Olasehinde, et al., an increase in the plates corrosion rate with a rise in temperature is the main reason for a decrease in the P. Dioica inhibition efficiency in the corrosive medium [10].
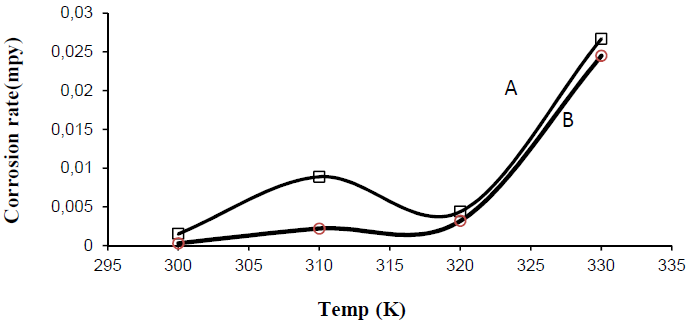
Figure 4 Steel surface corrosion rate variation in 100 mL water, without and with pulverized P. Dioica leaves optimum concentrations, at different temperatures. [A: blank solution, B: 3 g/100 mL tap water].
In the present study, we found that the corrosive medium without inhibitor showed much higher corrosion rate than that of the inhibited solution. Temperature has significant effect on the corrosion rate, and inhibitor parameters, i.e. temperature, influence the P. Dioica stability and solubility in the corrosive media [11-12]. The allspice leaf powder solubility increases with a rise in temperature. Therefore, the molecules present in the inhibitor can reach the mild steel substrate surface very easily, enhancing the inhibition efficiency. From the Arrhenius plot, we can find out the activation parameters using the mathematical equation,
where Ea = apparent activation energy for corrosion process, T = absolute temperature, and R = universal gas constant. Activation energy parameter, Ea, is obtained from the graph slope, and it is shown in Fig. 5. From the graph, we can observe that an increase in the activation energy is due to P. Dioica leaves powder physisorption onto the substrate surface.
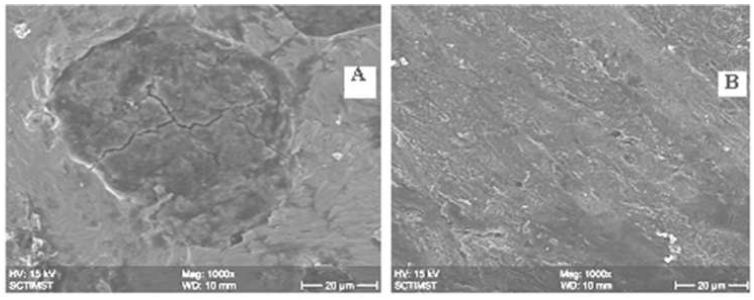
Figure 6 SEM images of mild steel coupons with and without inhibitor: [A: 0 g/100 mL water; B: 3 g/100 mL water; Temperature: 32 ºC].
Microscopic evaluation
Fig. 6 shows the SEM images of the mild steel specimens immersed in water, in the presence and absence of optimum inhibitor concentration of 3 g, for 5 days. It is clearly seen that the sample surface which was immersed in water, without inhibitor, shows higher significant corrosion than that of the sample kept in the corrosive medium with inhibitor.
Conclusion
The P. Dioica leaf powder green corrosion inhibitor influence on mild steel coupons in potable water was studied, and the results were discussed. Surface topography of the substrate coupon, before and after the corrosion study, was examined by scanning electron microscopic analysis, and found to be better than that of the bare coupons. Mass loss measurement and electrochemical analysis were conducted in potable water with different pulverized P. Dioica leaves concentrations. The green inhibitor efficiency increased with higher concentrations, but, after a particular concentration, it decreased. Optimum obtained leaf powder concentration was 3 g/100 mL.