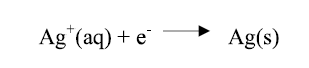Introduction
Ag is a precious metal that exists in the earth crust in very limited concentrations 1,2. Its unique and good electronic, optical, catalytic, electrical and antimicrobial properties explain its diverse uses in different sectors, such as electronics, biology, medicine, jewelry and photography 3-6. This versatility is responsible for the regular increase in Ag overall demand, which is about 2% per year 7. Ag is widely used in mirror industry, as a support layer responsible for reflection.
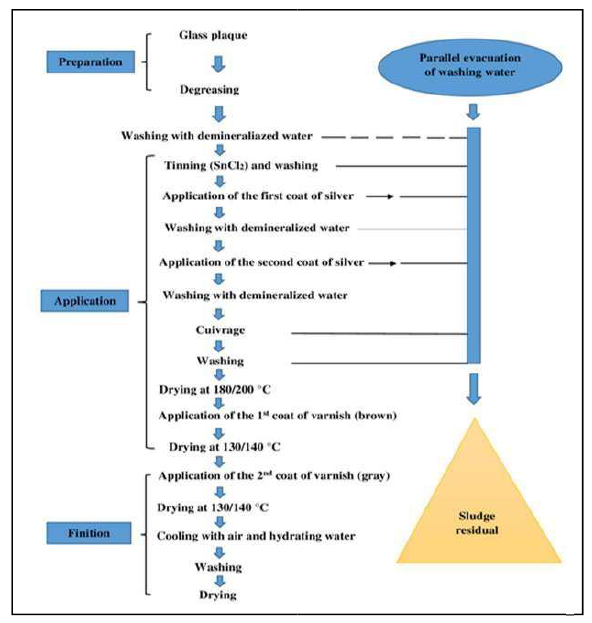
The mirror surface manufacturing process consists of the following main steps: surface preparation, mirror layer and protective coating application. In the first stage, the surface was washed. Then, it was polished, as the dust was cleaned with mechanical brushes, using a cerium dioxide suspension. After that, the sensitization process was carried out by applying a tin chloride (Cl-) solution. Then, the glass was rinsed and introduced into the activation surface phase, by pulverizing a palladium chloride (PdCl2) solution onto it. The rinsing was done again. The prepared glass was sent to the silvering chamber, where the Ag solution and the reducing agent were applied.
After the surface was prepared for painting, rinsing and drying, the mirror was covered with a protective paint layer, and dried at high temperatures, forming aqueous Ag waste 8,9.
This wastewater contains a high quantity of metal ions that exceed the limits of industrial effluent discharges established by agencies and applicable regulations. Therefore, it must be treated before discharge, due to its toxicity 10-13.
Ag industrial waste is a major source of environmental pollution 14. For example, in Algeria, according to the Official Journal of the Algerian Republic, N° 36, which was published on 21 June 2009, any harmful substances content of non-domestic waste water may not, under any circumstances, exceed the maximum limit value for Ag ions (0.1 mg/L), when they are discharged into a public sewage system or a wastewater treatment plant 15. Also, for economic and environmental reasons, Ag recovery has been of high importance. Conventional techniques for the removal of heavy metals and their solutions from wastewater, such as chemical precipitation, ions exchange, flocculation, cementation, coagulation, etc., have been used 16-25. Additionally, a series of promising studies and research has been carried out to recover Ag from these wastes, such as electrochemical processes 26-29. These methods have both advantages and limitations. Electrolysis is one of the most common available techniques, efficient for recovering Ag from industrial effluents. In this work, we have chosen this method due to its advantages of being clean, not requiring a lot of chemicals, and producing deposits with great purity 30.
The purpose of this work was to study the effect of CI, ET, V and T on Ag electrodeposition from the mirror effluents of the company Transformation et Vent Tous Types de Verre, Sarl El Hadj Com, El-Eulma, Sétif-Algeria. After that, parameters optimization was carried out. We have also recorded the solution pH and electrical conductivity (EC), before and after electrolysis. EC decreased with lower Ag ions amounts in the electrolysis.
Experimentals
Sampling
The samples of mirrors effluents containing Ag were taken after the silvering operation from the abovementioned company.
Technological chain of glass mirror manufacturing
To achieve the finished product, the mirror elements were subjected to a threephase treatment (preparation, application and finishing), as shown in Fig. 1. The successive coatings and abundant washes from the 2nd phase resulted in waste that was deposited, at the end of the operation, in the form of sludge. The Ag concentration in the sludge was 65%.
Chemical composition of the samples
The chemical composition of these samples was determined by the X-ray fluorescence technique (XRF), which is shown in Table 1. In this study, we used a solution that was removed from the effluent of the mirror company (February 2018).
Method
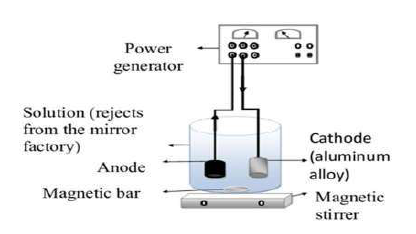
In this work, Ag has been recovered by the electrolysis technique. A two- electrodes cell containing an Al alloy, with a cross-section of 1 cm2, and graphite were used as working and auxiliary (WE and AE) electrodes, respectively. The Al alloy composition used for the electrodeposition studies was (Wt%): 80% Al, 17.9% Zn, 2% Mg and others in trace 32. Before each manipulation, the WE was mechanically pretreated by polishing it with an abrasive paper (grade 1200), chemically pretreated by ejecting it into the acetone for 10 min, rinsed with distilled water, and dried in the open air. Once the pre-treatment was completed, the WE and AE were immersed in a cell containing electrolytes (Ag wastewater) that have been connected to a power source (direct current), as indicated in Fig. 2.
Under the polarization effect, the electrons (ions contained in the solution) moved in opposite directions, so that the positively charged ions (Ag ions) moved towards the negatively charged cathode, being then neutralized by the electrons supplied by the cathode, and deposited as an Ag film, as shown in the equation below.
Once the electrolysis was completed, the WE was recovered, dried in the open air, and then weighed again after the electrolysis, to determine the deposited Ag mass, by the differential weight technique 33.
Results and discussion
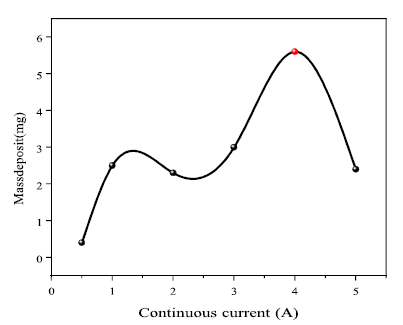
Continuous current effect on Ag electrodeposition
As shown in Fig. 3, the current was 4 A, the highest deposition value. Ag electrodeposition was favored by lower currents from 0 to 4 A. The electrodeposition amount increased with higher CI. In contrast, if the CI was over 4 A, the electrodeposition amount decreased. That may be explained by the high mobility of Ag ions in the solution, which prevented them from being deposited on the cathode surface.
Electrolysis time effect on Ag electrodeposition
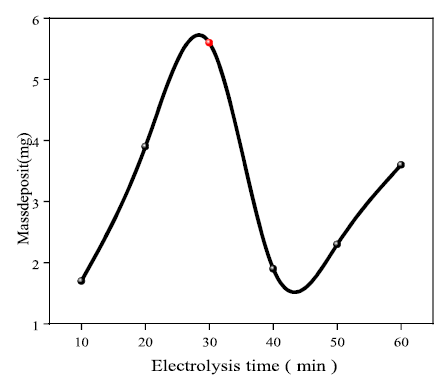
Based on the recorded results, it is seen that the ET had a positive influence on Ag electrodeposition, up to an optimal time of around 30 min. Over this period, the deposit mass rapidly dropped to 1.9 mg, and then marginally increased. This is probably due to the lower Ag mass transfer rate, for a long electrolysis period (see Fig. 4).
Voltage effect on Ag electrodeposition
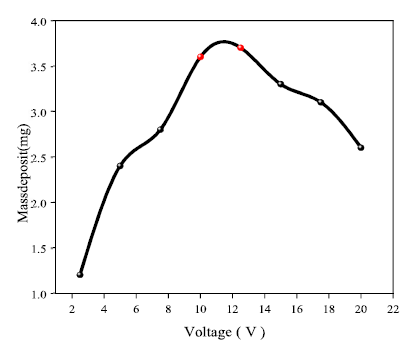
The results of the recovered deposit mass are shown in Fig. 5, with the increase in the voltage applied to Al, with a mass of about 3.7 mg, for an optimal value of 12.5 V. Indeed, the voltage forced the ions to migrate and deposit on the cathode. Values above 12.5 V decreased the Ag ions migration rate to the cathode and, consequently, the mass deposition was lower.
Temperature (T) effect on Ag electrodeposition
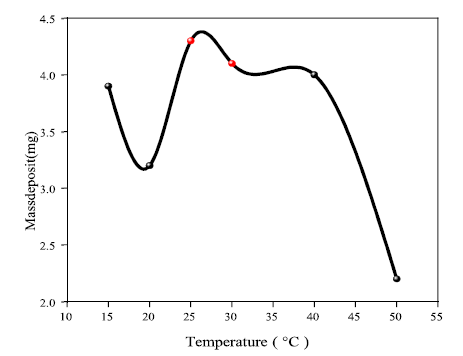
T is an important factor for electrochemical reactions in electrolysis 34. Indeed, it accelerates the electrodeposition rate, as shown in Fig. 6. This is valid for T around 30 ºC. On the other hand, T values higher than 30º have a negative effect on Ag electrolytic deposition. This could be due to the high thermal agitation that prevents Ag ions from being deposited on the Al electrode surface.
EC variation
According to the histograms a, b, c and d, in Fig. 7, the solution EC decreased after electrolysis.
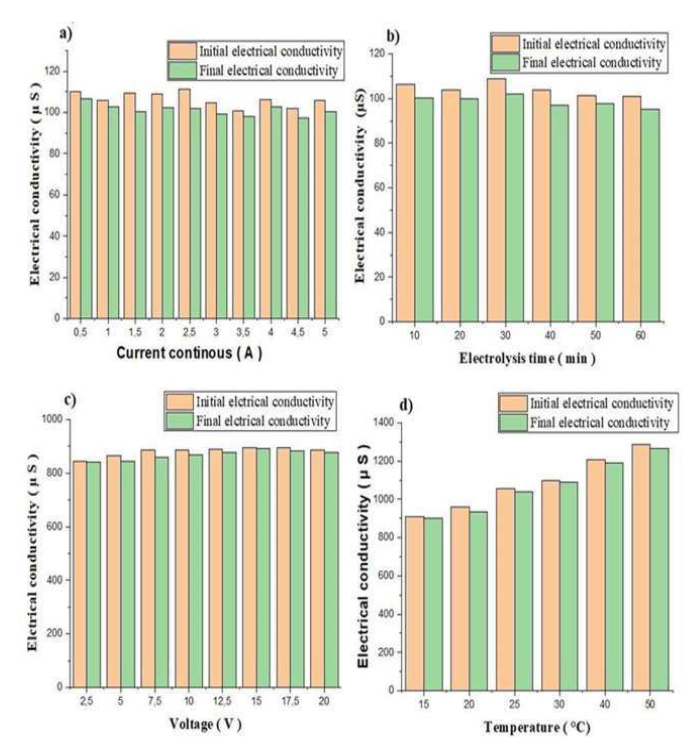
Figure 7 Variation in EC, before and after electrolysis, as a function of: a) continuous current, b) ET, c) V and d) T.
This can be explained by the decrease in Ag ions in the solution, which, after having been neutralized, were deposited as an Ag film. It was also seen that, with higher T, the ions rate in the solution increased, and, consequently, the EC was greater.
Ag deposits morphological analysis
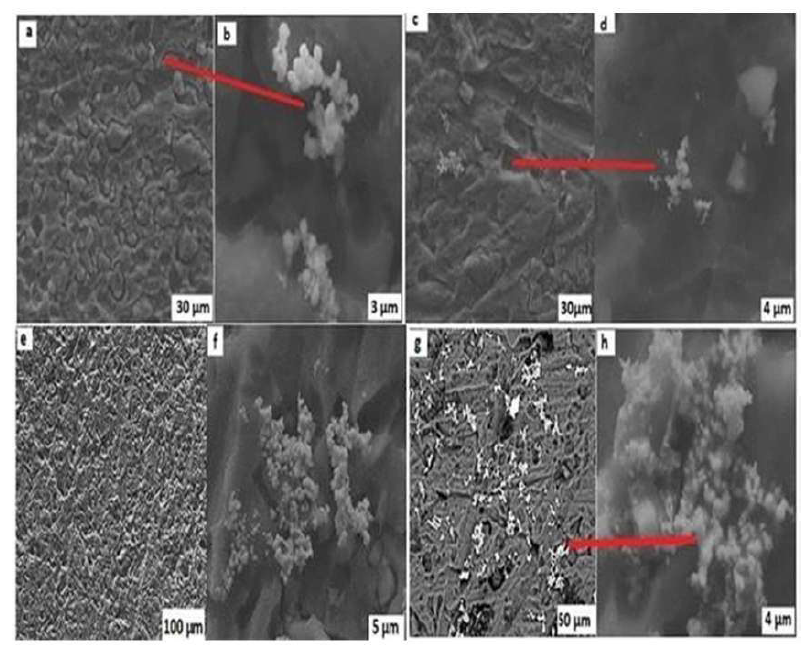
Fig. 8 shows the SEM image of Ag deposition obtained at different optimized electrolysis parameters. A dense deposit was formed on the Al electrode surface, in different amounts. To study the Ag aggregates morphology under different optimized parameters, we initially have set 4 A as the optimal DC current (a and b), at 12.5 V (c and d), the ET of 30 min (e and f), and T of 30 ºC (g and h). SEM images in Fig. 8 show that two essential steps, the Ag cations enrichment and reduction on the Al electrode surface, as highlighted by Zhao et al. 35, governed the Ag deposition mechanism. Similarly, the growth of Ag aggregates resulted in several layers where the surface film has gaps of different sizes (a, c, e and g), on which other Ag cations were enriched after their reduction at the electrode interface, to form flowers terminating in nodules (b, d, f, h). These results confirm those obtained by T. Nawaz et al. 36. According to these authors, the flowers collected on the gaps are due to Ag0 particles.
Conclusions
The aim of this work was to study the effect of CI, ET, V and T parameters on Ag electrodeposition over an Al electrode surface. Ag cations electrodeposition seems to be an efficient technique for recovering this metal from a residual sludge of the mirror industry. Optimization of these parameters resulted in a multilayer rich Ag deposit on the Al surface, followed by a cluster of flowers with different sizes relative to the reduced Ag0 observed by SEM.