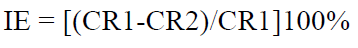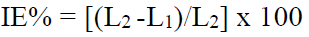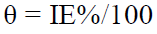Introduction
Mill scale is well removed by pickling, since the pickle liquor acids easily penetrate beneath its surface discontinuities, dissolving and removing the layer onto the base metal. Care should be taken to diminish metals time of immersion in the pickle solutions, as they may damage them. Pickling can be performed with a series of acids. In the contemporary pickling process, the most used is HCl, also known as muriatic acid. H₂SO₄ can also be used, even though it is less widely employed, since it can rapidly degrade the base metal. Acetic acid is another acid used now and then.
When corroded metal specimens are immersed in HCl, they will decay. To prevent this, many corrosion inhibitors have been employed in acidic media, which are discussed in this section.
Boumezzourh et al. [1] found a novel solution against food cans corrosion. The inhibition effect of Crocus Sativus flower aqueous extract on tinplate corrosion, in 0.5 M oxalic acid, was evaluated by gravimetric, electrochemical, SEM and EDX analyses. The results revealed that the extract reduced the corrosion action proportionally to its concentration, and reached maximum IE of 87.3%. SEM and EDX confirmed the oxygenated molecules adsorption onto the tinplate surface and its considerable improvement, due to the extract presence, compared with that exposed to the blank acidic medium.
Hassan and Ibrahim [2] used gasometric and WL methods for studying Mg corrosion inhibition in HCl by MC polysaccharide, as a carbohydrate polymer. The two techniques produced comparable experimental data. MC adsorption onto the Mg surface was found to obey both Langmuir’s and Freundlich’s isotherms. The inhibitor effect on Mg surface corrosion was confirmed by FT-IR and SEM techniques, which substantiated the formation of an MC film covering the metal.
Epoxy resin has electron donating and attracting properties. Therefore, About et al. [3] used PHQ resin as a green inhibitor of CS corrosion in a 0.5 M H₂SO₄ solution. WL and electrochemical methods were employed. The CS surface was investigated by SEM, EDS and AFM techniques. The molecules were adsorbed by chemisorption.
Fouda et al. [4] investigated Ferula hermonis plant extract IE% against Zn corrosion in a 1 M HCl solution. The study used WL, PDP, EIS, EFM and surface examination analyses. The extract biological action was studied. The results indicated that there was a protective film formed by the inhibitor on the Zn surface, which protected it from dissolution in the HCl medium.
Chen et al. [5] used expired vitamin B1 drugs to control AA5083 aluminum alloy corrosion in a HCl solution. WL method, PDP, EIS and surface analyses have been used. The results specify that IE% increased with higher inhibitor concentrations, at several temperatures, reaching its maximum value of 87.85%, at 288 K.
API 5L X52 steel corrosion inhibition, in a 1 M HCl medium, by 1,2,3-triazole derived from BnOH, was studied by Espinoza Vázquez et al. [6]. BnOH and all its synthesized derivatives fitted the Langmuir’s adsorption isotherm. The shared adsorption phenomenon was anticipated according to the obtained thermodynamic parameter values. The observed physisorption-chemisorption was explained in terms of the electrostatic interactions and chemical bonding amidst the metal surface and the corrosion inhibitor [6].
Zhang et al. [7] used ATKPE as a green inhibitor for MS corrosion in HCl solutions. ATKPE chemical composition was analyzed, and its IE% was investigated by electrochemical methods, surface analysis and hypothetical calculations. Electrochemical measurements showed that ATKPE is a mixed-type corrosion inhibitor that obeyed Langmuir’s adsorption isotherm. In addition, SEM, AFM and contact angle observations suggested that ATKPE may attach tightly to the metal surface by forming a barricade film.
The fluids used in oil wells acidification aggravate metallic materials corrosion undeniably. As a result, corrosion inhibitors are desirable to mitigate or stop the corrosion process.
Ricky et al [8] used C21H36O as inhibitor for MS corrosion in a 1 M HCl solution. They reported that C21H36O, which is produced from cashew nut shell liquid, is an eco-friendly, low-cost and sustainable inhibitor. Thus, it can replace non-green and expensive corrosion inhibitors used in the industry.
Al Kiey et al. [9] reported the potential inhibitive performance of green and sustainable inhibitors based on cellulose derivatives against CS corrosion. CTZ was synthesized and tested as a benign inhibitor for CS corrosion in a 1 M HCl solution. FT-IR, SEM, XRD and TGA were used to analyze the synthesized CTZ. The inhibitor mechanism was further investigated using a diversity of techniques, including WL, PDP and EIS.
Shahmoradi et al. [10] reported theoretical and surface/electrochemical investigations of walnut fruit green husk extract as an effective inhibitor for MS corrosion in a 1 M HCl electrolyte. The extrapolated Tafel polarization results revealed that the extract functions as a mixed-type corrosion inhibitor. In addition, XPS, FT-IR, SEM, AFM and UV-visible spectroscopy were used to study the inhibition mechanism of the agricultural waste extract in the HCl medium. Quantum chemical calculations based on DFT and molecular dynamic simulations were carried out on the adsorbed Juglone inhibitor.
Experimental
The present work aimed to investigate the inhibitive effect of a seaweed alcoholic extract (SM) on MS corrosion in 1 M HCl (immersion period of 30 min), by WL and PDP. The protective film was analyzed by AFM and Vickers hardness test.
MS specimens
The MS specimens used for WL and surface examination studies were composed of C (0.079%), P (0.025%), Mn (0.018%), S (0.021%) and the remainder Fe, with the dimensions of 4.0 x 1.0 x 0.2 cm.
For the electrochemical technique, these MS specimens, encapsulated in Teflon, with an unprotected area of 1 cm2, were used. Before each experiment, the metal specimens were polished to mirror finish by different grades of emery sheets, washed with double distilled water, degreased by acetone, air dried and preserved. All the corrosive media (1 M HCl) were prepared by distilled water, and standardized.
SM extract preparation
SM (Fig. 1) [11] was collected from Ramanathapuram. The seaweed was air dried, powdered and weighed. The extract was prepared by refluxing 5 g of the powdered seaweed in an ethanol medium, for 8 h. After one day, the extract was filtered and placed in an air tight container. SM, commonly known as Japanese wireweed, is a large brown seaweed of the genus Sargassum. It is an invasive seaweed with high growth rate. Due to its floats, it is easily dispersed. Details of the species are as follows [11]: Kingdom: Chromista; Species: S. muticum; Phylum: Ochrophyta; Family: Sargassaceae; Rank: species; Class: Phaeophyceae; Genus: Sargassum; Order: Fucales.
SM contains anti-fouling agents and anti-oxidant compounds [12].
Anti-fouling agents
Secondary metabolites produced by marine algae could constitute an interesting alternative antifouling agent. Previous studies have showed the potential of hydrocarbon and fatty acid compounds, such as galactolipids, palmatic acid, 1-tetradecene or 1-hexadecene, in antifouling activities. Moreover, the peak production of antifouling compounds is during the spring.
Anti-oxidant compounds
SM is rich in anti-oxidant compounds, such as phenolics (cathechins, phlorotannins and quercetins), pigments (fucoxanthin) and vitamins (C, K and E, in the form of alpha/gamma-tocopherols). Applications are possible in pharmaceuticals, cosmetics and health fields, due to the anti-oxidant activities of these molecules.
WL method
The three polished MS specimens weight was measured, before and after immersion for 30 min, in various test solutions (1 M HCl without and with different SM concentrations). SM IE% values were calculated from the relation:
where CR1 and CR2 are the corrosion rate without and with inhibitor, respectively.
Adsorption isotherms
There are various types of adsorption isotherms, such as Langmuir’s, Freundlich’s and Temkin’s. This study found that the SM molecules adsorption onto the MS surface obeyed Langmuir’s adsorption isotherm.
Electrochemical study
In the present work, MS resistance to corrosion in various acidic test solutions was measured by PDP. Electrochemical measurements were performed in an Ivium compact-stat electrochemical measurement unit.
PDP study
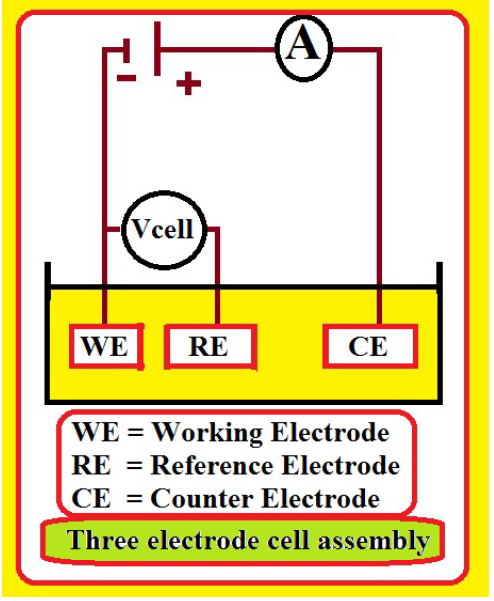
PDP studies were carried out in a three electrode cell assembly (Fig. 2).
SC, Pt and MS were the RE, CE and WE, respectively. From the PDP study, corrosion parameters, such as Ecorr, Icorr, ba, bc and LPR values, were measured.
Vickers hardness
The CS specimens immersed in various test solutions, for one day, were taken out, rinsed with double distilled water, dried and subjected to the Vickers hardness test that was carried out by Shimadzu make model HMV-27.
Results and discussion
WL method
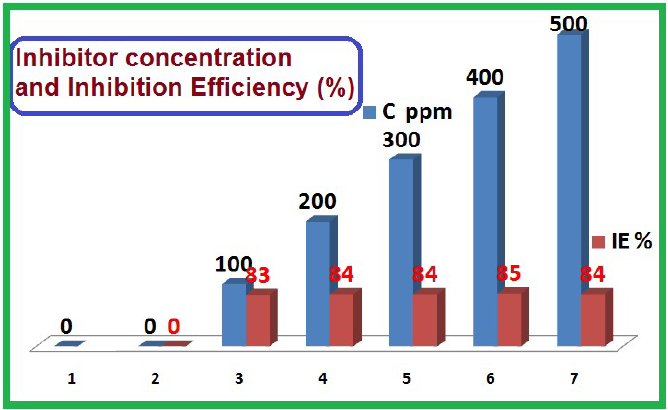
It was observed that the inhibitor system offered good IE% against MS corrosion in HCl. MS CR values, SM IE% (immersion period of 30 min), θ and C/θ values are given in Table 1.
Table 1 MS corrosion rates in 1 M HCl, for 30 min, and SM IE% values (immersion period of 30 min).
| Inhibitor conc. ppm | CR mdd | IE% | θ | C/θ |
| 0 | 25.83 | - | - | - |
| 100 | 7.49 | 71 | 0.71 | 141 |
| 200 | 6.46 | 75 | 0.75 | 267 |
| 300 | 5.86 | 78 | 0.78 | 385 |
| 400 | 4.91 | 81 | 0.81 | 494 |
| 500 | 4.13 | 84 | 0.84 | 595 |
Electrochemical studies
Electrochemical studies, such as PPD and AC impedance spectra, have been employed in corrosion inhibition studies [13-20]. When corrosion resistance increased because of the blocking (blanket) effect of the inhibitor molecules adsorbed onto the metal surfaces, the following observations were made: LPR increased and Icorr decreased. Rct, impedance and phase angle increased, and Cdl decreased (Fig. 3).
PPD study
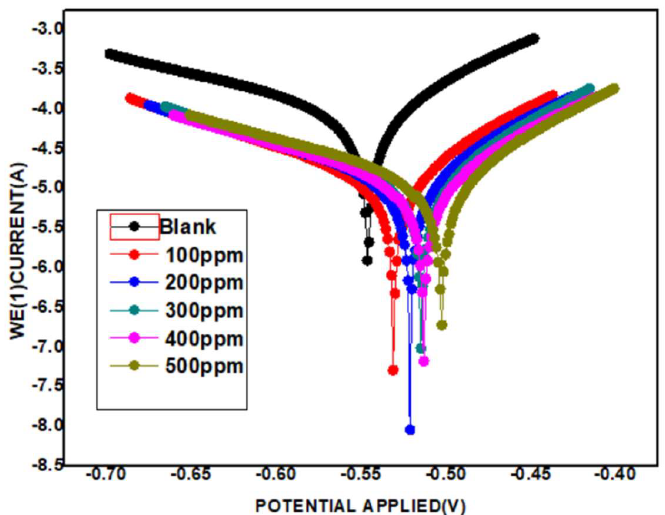
The polarization curves (Tafel plots) of MS immersed in 1 M HCl, without and with SM, are shown in Fig. 4.
The electrodes were immersed in the test solutions for 30 min, in order to reach the steady state potential.
Corrosion parameters of MS immersed (for 30 min) in 1 M HCl, with SM alcoholic extract, were obtained from the PDP study, and are given in Table 2.
Table 2 Corrosion parameters of MS immersed in 1 M HCl, obtained from the PDP study (30 min of immersion).
| Inhibitor conc. ppm | Ecorr mV vs. SC. | bc mV/decade | ba mV/decade | LPR Ohm/cm2 | Icorr A/cm2 |
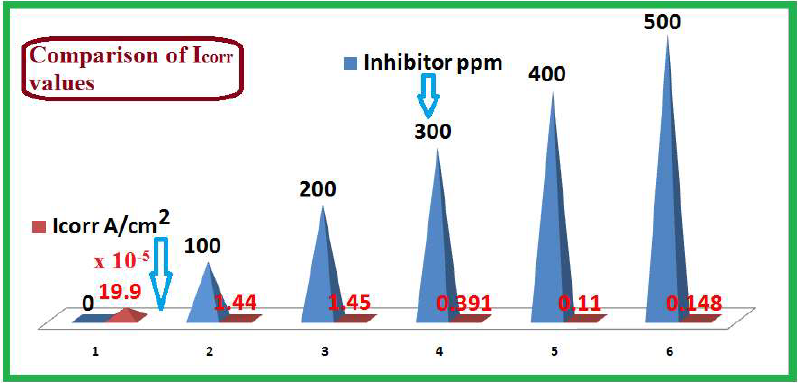
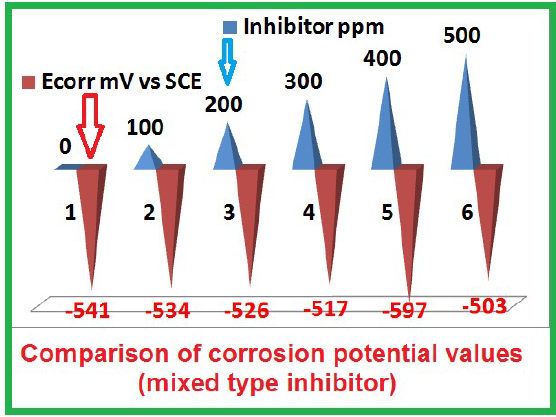
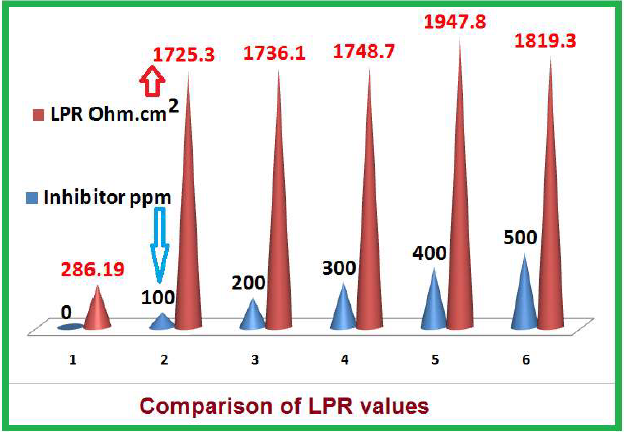
| 0 | -541 | 113 | 257 | 286.19 | 19.9 x 10-5 |
| 100 | -534 | 088 | 164 | 1725.3 | 1.44 x 10-5 |
| 200 | -526 | 196 | 196 | 1736.1 | 1.45 x 10-5 |
| 300 | -517 | 185 | 185 | 1748.7 | 0.391 x 10-5 |
| 400 | -597 | 205 | 205 | 1947.8 | 0.110 x 10-5 |
| 500 | -503 | 917 | 201 | 1819.3 | 0.148 x 10-5 |
Various SM concentrations were used. It was observed that, with the inhibitor, Ecorr shifted to the anodic side, in most cases. This suggests that the anodic reaction was predominantly controlled. Nevertheless, the shift was within 50 mV. Hence, it was inferred that the inhibitor is of the mixed type, controlling anodic and cathodic reactions to an equal extent.
This can be explained by the inhibitor blanket effect, which blocked the electrons transfer from the metal into the solution. Further, since the anodic and cathodic sites were blocked by the inhibitor molecules, the electrons could not be transferred from one to another. So, it was also observed that, with the inhibitor, LPR increased and Icorr decreased. With higher inhibitor concentrations, the blanket effect increased.
IE% calculation
The inhibitor system IE% was calculated from the PDP study, using the formula
where L1 and L2 are the LPR values, without and with inhibitor, respectively.
SM IE% values are given in Table 3.
Table 3 SM IE% calculated from LPR values.
| Inhibitor ppm | IE % |
| 0 | 0 |
| 100 | 83 |
| 200 | 84 |
| 300 | 84 |
| 400 | 85 |
| 500 | 84 |
Influence of SM concentration on I corr of MS in 1 M HCl
The influence of SM concentration on Icorr of MS in 1 M HCl is given in Table 5 and Fig. 5. It was observed that, with higher SM concentrations, Icorr decreased. Further, the shift in Ecorr of the solution with inhibitor was much slower than that of the blank solution.
Effect of the inhibitor concentration on E corr of MS in 1 M HCl
The effect of SM concentration on Ecorr of MS in 1 M HCl is given in Table 5 and Fig. 6. It was observed that, with higher inhibitor concentrations, Ecorr value slightly shifted to the anodic side, indicating that the anodic reaction was predominantly controlled. However, the shift was very small (within 50 mV), which implies that the inhibitor is of the mixed type, controlling both anodic and cathodic reactions. This explains the SM IE% increase with higher concentrations.
Influence of the inhibitor concentration on LPR of MS in 1 M HCl
The influence of the inhibitor concentration on LPR of MS in 1 M HCl is given in Table 5 and Fig. 7. It was observed that, with higher concentrations, LPR value, and hence, SM IE%, increased. This is due to the fact that the protective film formed on the MS surface blocked the electrons movement from the anodic to the cathodic sites, and also from the metal to the solution. This is also supported by the fact that the inhibitor is of the mixed type, controlling both anodic and cathodic reactions.
Effect of the inhibitor concentration on its IE% of MS corrosion in 1 M HCl
Influence of SM concentration on its IE% of MS corrosion in 1 M HCl is shown in Table 5 and Fig. 8. It was observed that, with higher inhibitor concentrations, LPR increased, Icorr decreased and hence, SM IE% grew. This is further supported by the fact that the inhibitor is of the mixed type. In addition to this point, with higher inhibitor concentrations, more of its molecules are transported to the MS surface and are adsorbed onto it, thus enhancing SM IE%.
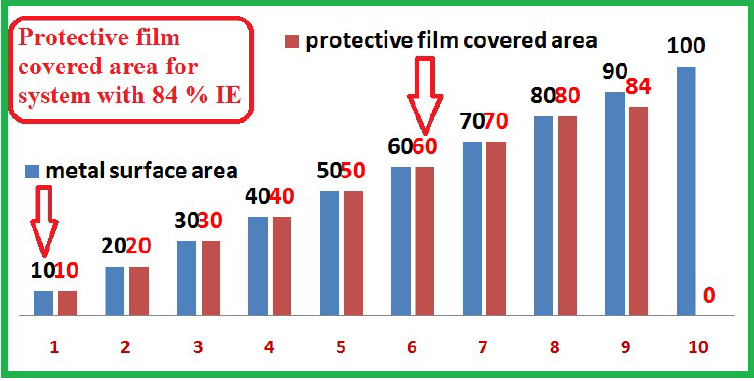
SM protective film that covered MS area had a corrosion IE% was 84% (Fig. 9).
Langmuir’s adsorption isotherm
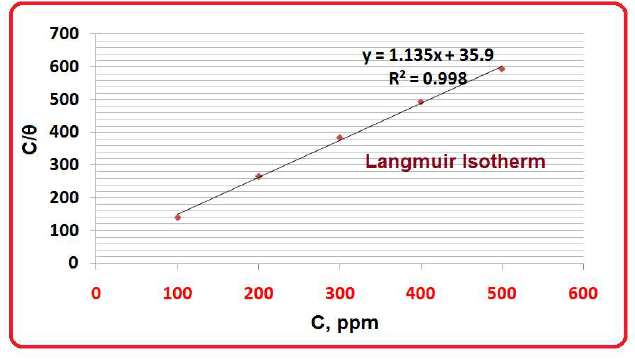
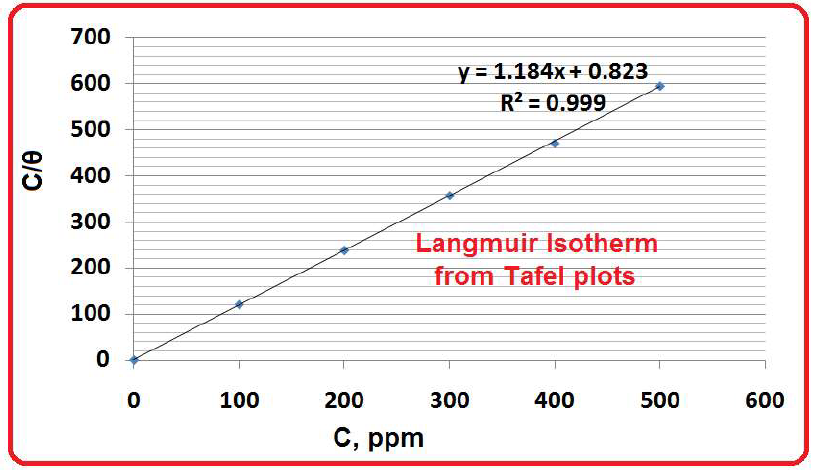
The Langmuir’s adsorption isotherm is used to describe the equilibrium between adsorbate and adsorbent system, where the adsorption of the former is limited to one molecular layer. In this study, the blocking effect (blanket) of the inhibitor adsorbed molecules was taken into account. It was responsible for SM (active principles present in the seaweed alcoholic extract) corrosion IE%, whereby the inhibitor molecules were adsorbed as monolayers onto the MS surface (Table 4 and Fig. 10).
Table 4 Parameters for Langmuir’s adsorption isotherm.
| C ppm | C/θ |
| 100 | 141 |
| 200 | 267 |
| 300 | 385 |
| 400 | 494 |
| 500 | 595 |
From Langmuir’s isotherm, a plot of C (inhibitor concentration in ppm) vs. C/θ gave a straight line, and by the following relation:
The parameters needed for the plot of Langmuir’s adsorption isotherm are given in Table 5. θ is of one inhibitor molecular layer, since multilayers are not possible. R2 value (Fig. 11) was very high (0.999).
Table 5 Parameters needed for the plot of Langmuir’s adsorption isotherm (from the polarization study).
| Inhibitor ppm | C/(θ) |
| 0 | 0 |
| 100 | 121 |
| 200 | 238.1 |
| 300 | 357.1 |
| 400 | 470.6 |
| 500 | 595.2 |
Conclusions
SM extract effect on MS corrosion control in 1 M HCl was evaluated by the WL method and electrochemical techniques, namely, PDP.
The protective film was investigated by the Vickers hardness test.
WL method revealed that 500 ppm SM offered 84% IE against the corrosion of MS immersed in 1 M HCl.
SM molecules adsorption onto the metal surface obeyed Langmuir’s adsorption isotherm. The R2 value was very high (0.999).
PDP study revealed that the Icorr slightly shifted to the anodic side. It was inferred that the inhibitor solution is of the mixed type, since the shifts were relatively small.
With SM, LPR increased and Icorr decreased. Because of the blanket effect, the electrons transfer from the metal to the bulk of the solution was blocked.
It was found that the inhibitor formed a protective film monolayer.
Vickers hardness from the inhibited MS surface was lower than that of the polished one, but higher than that of the corroded one.
The outcomes of the study may be used in the pickling industry [21], where HCl is used to simultaneously remove the rust from metals surfaces and protect them.
Authors’ contributions
V. Jeslina: collected the data. S. Jone Kirubavathy: collected the data; made the paper English correction. Abdulhameed Al-Hashem: improved the quality of the paper; was an international collaborator. S. Rajendran: conceived and designed the analysis; wrote the paper; acted as corresponding author and coordinator. R. M. Joany: inserted data or analysis tools; performed the analysis. Caslav Lacnjevac: inserted data or analysis tools; wrote the paper; was an international collaborator.
Abbreviations
AC: alternating current
AFM: atomic force microscopy
ATKPE: Akebia trifoliate koiaz peels extract
ba: anodic Tafel slope
bc: cathodic Tafel slope
BnOH: benzyl alcohol
C21H36O: M-pentadecylphenol
Cdl: double layer capacitance
CE: counter electrode
CR: corrosion rate
CS: carbon steel
CTZ: cellulose tetrazole
DFT: density functional theory
Ecorr: corrosion potential
EDS: energy dispersive spectroscopy
EDX: energy dispersive X-rays
EFM: electrochemical frequency modulation
EIS: electrochemical impedance spectroscopy
FT-IR: Fourier-transform infrared spectroscopy
H2SO4: sulphuric acid
HCl: hydrochloric acid
Icorr: corrosion current density
IE%: inhibition efficiency
LPR: linear polarization resistance
MC: methyl cellulose
MS: mild steel
PDP: potentiodynamic polarization
PHQ: Pentaglycidyl ether pentaphenoxy of phosphorus
R2: coefficient of determination
Rct: charge transfer resistance
RE: reference electrode SC: saturated calomel
SC: saturated calomel
SEM: scanning electron microscopy
SM: Sargassum muticum
TGA: thermal gravimetric analysis
WE: working electrode
WL: weight loss
XPS: X-Ray photoelectron spectroscopy
XRD: X-ray diffraction
θ: surface coverage