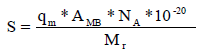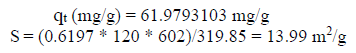Introduction
WO contains many elements that are toxic to health and the environment, such as heavy metals, organic acids, phenols, phthalates, PAH, etc. Naturally, these oils are far from being biodegradable, and must therefore be recycled for reuse, or transformed into industrial fats 1. WO discharge into the wastewater network is a real ecological disaster. Once discarded, they clog filters at treatment plants, and disrupt biological purification processes. 1 L of WO can cover 1000 m2 of water, and hinder the oxygenation of underwater flora and fauna for years. Sources of used motor oil are classified according to four consumer categories: large industry; small urban businesses, such as garages and service stations; agricultural and rural sectors; and private individuals who drain their vehicles. Industry and mechanics produce the vast majority of WO 2.
To cope with this problem, several recycling processes have been put in place, which are based on the filtration of BT as a drying layer and adsorbent of fine particles and vesicles. Thus, non-negligible quantities of waste BT soiled with WO residues treated in the form of cakes in the filter press are generated at the end of the cycle 3. WO treatment stages are herein presented in detail (Fig. 1). Then, soiled BT permeability and its parasitology were studied, in order to assess if it is doable to reuse the sludge cake containing impurities from WO impregnated in BT, as floor covering in controlled landfills. It is for this purpose that the present work was focused on the valorization of sludge recycling from used lubricants 4.
Before proceeding with the study of soiled BT, it was necessary to analyze it in the raw state. This study aimed to investigate BT-WO residues PC properties, such as permeability, specific surface, adhesion to soil, parasitology and toxicology, in order to determine their possible uses in the valorization process 5.
Materials and methods
BT and WO general characteristics
BT
BT are, by definition, clayey materials essentially composed of smectite (which include montmorillonite). From the genetic point of view, the main deposits of BT are generally derived from the transformation in place of glassy volcanic ash, in an aqueous medium, or from the hydrothermal alteration of volcanic rocks. Certain layers of sedimentary clays, rich enough in smectite, can also constitute BT deposits 6. From an economic point of view, the distance between the exploitable deposit and the place of consumption can reach several thousand kilometers. For open pit deposits, the D:E thickness ratio can be high, reaching 10 km. The minimum size of an exploitable deposit is in the order of 200 000 t.
BT are mainly used for: animal feed; degrease and bleach products, in civil engineering; sails sealing; cement injections; muds drilling; manufacture of foundry molds; iron ore pelletizing; and soil amendments. The content selection criteria are as follows: smectite > 70%, sand < 30% and calcite < 10%.
BT origin
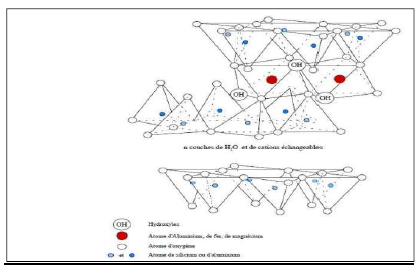
BT was discovered for the first time in 1847, near Montmorillon, in the region of Vienne (France). BT are clays of volcanic origin, consisting mainly of montmorillonite (more than 75%) (Fig. 2). The alteration and hydrothermal transformation of ash from glass-rich volcanic tuffs leads to the neoformation of clay minerals, which are part of the smectite group 7. BT is a denomination of montmorillonite 8.
BT structure and composition
In its natural, raw form, BT is a soft rock with about the consistency of kaolin, i.e., friable, unctuous to the touch, and its hue is white, gray or slightly tinged with yellow. It comes from the devitrification of volcanic layers under the influence of alkaline or acidic waters. Besides montmorillonite, this rock may contain other clay minerals (e.g. kaolinite, illite), as well as impurities in the form of gypsum, carbonates, etc.
WO characteristics
Lubricating oils are viscous liquids used for the lubrication of engines and machines mobile parts 9.
Studied industrial waste
BT-WO cakes were taken from a used oil treatment unit 10.
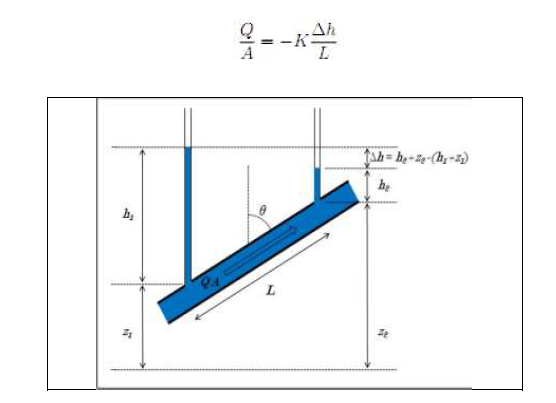
Notions of permeability (Darcy's Law)
If a water pressure gradient, Δh/L, is applied to a porous medium that is completely saturated with water, the water will flow through the connected pores (Fig. 2).
It is assumed that the average flow rate, Q/A (the total flow rate), is proportional to the pressure gradient, Δh/L (Darcy, 1856) (Fig. 3).
Biosorbent preparation
On WO delivery in the treatment unit, either by tanker truck or in hermetically sealed 200 L metal drums, a pumped transfer operation to the storage and settling basin takes place. Then, a second transfer is made to the heat-treatment tank (temperature: 150-170 ºC). Heating is provided by a coil heated with fuel that circulates in a closed loop 11. The heating operation lasts about 2 h. The second operation, which is not always compulsory, and depends on the clientele specifications, consists in carrying out a PC treatment by adding concentrated H₂SO₄ (beer: 60; density: 1.84; and mixing ratio: 0.2 - 0.3%) to thermally treated oil 12. Stirring thereof for about 20-30 min will lift the emulsion and separate water from oil. The third operation is to circulate the oil that has undergone the first two operations through a bed of BT, in order to absorb the vesicles and dehydrate it. The last operation allows to separate the treated oil from the soiled BT, with a filter press 13. BT is compacted into cakes in the pressed filter walls. The oil freed from its impurities is recovered in the metal drums, or directly transferred to the tanker truck. The unit processes about 3 tons per day of WO, and produces about 1 ton of BT waste (Fig. 4).
Used BT-WO permeability and specific surface study
Soiled BT physicochemical study
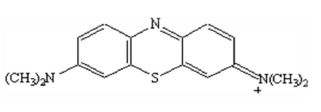
The dye used was cationic MB (Fig. 5). Its crude formula is C16H18N3S. MB is applied in textile industry (wool, leather, silk, etc.). It is a color marker for DNA cells, and serves as a redox colored indicator. It has a λmax of 665 nm and a molar mass of 373.9 g/mol. MB calibration absorbance equation is 0.145 x C 0.096. The solution was prepared by the dye dissolution through distilled water.
MB calibration curve determination
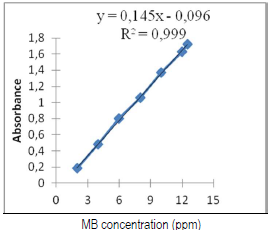
Before starting adsorption tests, the stability and the linearity range of the MB solutions and vegetable waters must be evaluated, and the results graphically presented. No significant difference was observed in the absorbance that was measured twice, at t = 0 and 60 min, for each tested concentration. As a function of concentrations, MB very good absorbance linearity (λmax = 665 nm) was found. Calibration was carried out for the solutions with the following concentrations: 1, 3, 5, 8, 10 and 12 ppm. We plotted the absorbance as a function of concentration: A = f (C) (Fig. 6).
From the MB calibration curve, we obtained the following equation: A = 0.145 C - 0.096. After determining the wavelength and the calibration curve, we used tap water contaminated with the dye, in order to simulate a sample of effluents from the textile industry. Note that Beer and Lambert's law is valid for MB concentrations from 1 to 12 ppm (0.1 < A< 2).
The adsorption capacity (qe) of the solid phase solute fraction was deduced by the material balance, where MB: C0V = m0 * qe + Ce V; qt = (C0 - Ce) * V/m0. The reactor in the batch mode was: qt (mg/g) = 61.9793103.
Specific surface
The knowledge of the specific surface is paramount in the characterization of a powder or an aerosol, whatever the field of application. Its determination helps to know the sample reactivity.
The physical principle, universally recognized for the specific surface determination, is based on liquid adsorption. It allows: the measurement of the sample geometrical texture without modifying it; and the determination of the particles surface total area, including the surface of the open pores or creeks in cul-de-sac, accessible to the external liquids molecules 14.
This adsorption phenomenon occurs by means of the so-called weak or secondary forces (Van der Waals’s forces) on the particles surface. These forces act outwards, in particular on liquid molecules that surround the sample under analysis, whatever the chemical nature of the body present.
The specific surface area of the collected sample is determined by two methods.
BET method
BET theory allows, from the results obtained, to determine the aerosol specific surface area. This technique enables to determine the amount of adsorbable N on the surface of a powdery compound. N porosimetry was measured using a Quantasorb Junior device (ANKERSMITH) 15.
MB method
The principle of this method consists in determining the required MB quantity, to cover a monomolecular layer of the fine particles outer and inner surfaces of a solid suspended in water 16.
A mass of about 1 g of a finely ground sample was mixed in a 100 mL beaker with 20 mL distilled water, continuously stirring for a few min. Then, the mixture was metered with a MB solution of a known mass concentration (drop by drop), until the light blue halo which surrounded the spot central deposit was formed on the filter paper.
Measurement of the specific surface area (MB method)
The specific surface area 17 is given by the following relation:
where qm is maximum adsorption capacity (mg/g), AMB is the area occupied on average by a MB molecule, which equals 120 A°2, NA is Avogadro’s number (6.02.1023 mol-1) and Mr is MB molar mass (319.85 g/mol-1).
The analyses carried out in the laboratory, based on Darcy’s law, showed that the charged BT cake was impervious. Table 1 shows the results obtained while comparing BT WO properties with those of raw clay 18.
Table 1 Raw BT and BT WO characteristics.
| Characteristics | Raw BT | BT WO |
|---|---|---|
| Porosity (mm3/g) | 156,7 | 236 |
| Specific surface (m2/g) | 30.8 | 13,99 |
| Permeability kf x 10-10 (m/s) | 2.1 | < 1 |
Bacteriological study of BT soiled with WO
Sample general characteristics
In order to study the bacteriology of a BT sample resulting from recycled WO (Table 2), bacteriological tests on 3 samples, of which manipulation is detailed in the following points (Fig. 7), have been carried out.
Compound A preparation and characterization
The sample preliminary preparation was done through compound A solubilization, putting 12 g of it in a 100 mL CHCl3 solution, for centrifugation, in order to separate the pellet. The pellet was taken up in water, and the supernatant was centrifuged, for 1 h, at 14000 rpm (40 ºC), in a rotavapor, to remove CHCl3 (Fig. 8), which was the aqueous phase, and keep the organic phase (oil) 19.
Table 3 shows the protocol followed for preparing solutions with the following characteristics.
Table 3 Characteristics of prepared solutions.
| Solution 1 | Solution 2 | Solution 3 |
|---|---|---|
| 1,5 g pellet + 250 mL TW water | 10 mL solution + 240 mL TW | 30 mL oil + 220 mL TW water |
Then, three petri dishes were prepared for germinating 15 g barley and lentils, which were sprayed with 3 types of solutions, as shown in Table 4 and Fig. 9.
Table 4 Petri dishes characteristics.
| Petri dishes /day | Petri 1 | Petri 2 | Petri 3 |
| Watering | TW | Solution 1 | Solution 3 |
| (day 0) | - | - | - |
| After 2days | + | + | - |
| After 3 days | +++ | ++ | - |
| After 5 days | +++ | +++ | + |
No germination: -; germination: ++; start of germination: +; and prolonged germination: +++.
Results and discussion
According to the tests carried out in the laboratory for determining BT permeability and specific surface area, it was found that it was impermeable 20.
BT germination and bacteriology studies showed that the germination was accelerated in box 2 (containing the pellet), while it was too slow in box 3 (containing oil). This was due to the supernatant, which inhibited germination 21), (22.
Soiled BT toxicology study
Tests on mice and rats
In order to study the toxicity of the pellet and supernatant, which are sample A solubilization residues 23, tests were carried out on rats and mice, and on Tp (Table 5).
The mice were divided into 5 groups: 1. (control reference): administration of 0.9% NaCl; 2. feeding with 60 μL supernatant; 3. feeding with 60 μL pellet; 4. intraperitoneal injection of 60 μL supernatant; and 5. intraperitoneal injection of 60 μL pellet.
Table 5 Tests on mice.
| Groups/days | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|
| First day | 23,4 | 23,8 | 22,9 | 24,2 | 23,0 |
| Third day | 26,6 | 24,3 | 23,1 | 22,8 | 21,4 |
| Seventh day | 28,2 | 25,5 | 24,4 | 20,09 | 21,3 |
| Ninth day | 29,3 | 26,0 | 26,87 | 21.0 | 21,0 |
| After tenth day | 32,4 | 26,7 | 27,0 | 18,1 | 19,0 |
The mice treatment did not cause a lethal effect, but decreased their weight in the groups injected with the supernatant and the pellet 24. The pellet and the supernatant did not cause inflammatory reactions, since no redness (erythema) neither swelling (edema) were seen in the in rats subjected to daily application for one week.
Test on Tp
In a culture medium of 1.2% peptone proteose and 0.25% yeast extract seeded with 1% Tp culture 25, increasing volumes of supernatant and pellet were incubated for 72 h. After incubation, counting was made under a microscope (Fig. 9 and Table 6) 26.
Table 6 Tests on Tp and control tubes
| Tubes | T | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Vs/c (µL) | 0 | 5 | 10 | 20 | 50 | 100 | 200 | 500 | 800 | 1000 | 2000 |
| Tp (µL) | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| MC (mL) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| S | + | + | + | + | + | + | + | + | - | - | - |
| C | + | + | + | + | + | + | - | - | - | - | - |
No growth: -; with growth: +
It was noted that, with pellet and supernatant low concentrations, Tp multiplied in a similar manner as in the control tube, whereas, with high concentrations, growth decreased until it started declining 27. The supernatant was directly used in a pellet solution that was prepared at the rate of 3 g in 40 mL distilled water 28.
Conclusion
Based on the results presented in this work, it is clear that the employed recovery method allowed the reuse of BT-dough as a soil-tight coating, with characteristics close to those of the others. In the recycling process of WO, the final step was to pass them through a filter press containing BT. The WO was treated with the following steps: heating at 90 ºC; acidification with H₂SO₄; and neutralization with soda. The pressed cake was then left out in the wild, or transported to the landfill. The study focused on the reuse of these BT mud cakes soiled with impurities from recycled WO. Physicochemical and toxicological studies on the pulp properties enabled to recommend the reuse of this slurry mud as a waterproof and very suitable soil covering. Thus, controlled landfills, sports fields and irrigation water storage basins can be coated with this compact clay, in order to prevent infiltration into the water table. The present work was articulated around two main parts. The first concerned the study of the material specific characteristics (BT extracted from WO): permeability, specific surface, porosity, adhesion to the ground and durability. The second concerned the bacteriological and eco-toxicological study of BT-WO material, in order to prevent all the negative impacts on the soil quality, groundwater, vegetation and human health.
Acknowledgments
The first author of this paper acknowledges all the persons that gave any information and remarks to make this work succeed, and also all the staff of the Laboratory of Engineering Process and Environment.
Authors’ contributions
Mohammed Tahiri: collected the data; wrote the paper. Said Akazdam: collected the data; inserted data or analysis tools; wrote the paper. Hajar Quachach: performed the analysis. Noureddine Bourhim: conceived and designed the analysis. Mohammed Loutfi: performed the analysis. Mohammed Chafi: performed the analysis; wrote the paper.
Abbreviations
BET: Brunauer-Emmett-Teller
BT: bentonite
CHCl3: chloroform
D:E: discovery/exploration ratio
H₂SO₄: sulphuric acid
MB: methylene blue
NaCl: sodium chloride
PAH: polycyclic aromatic hydrocarbons
PC: physiochemical
Redox: reduction-oxidation
Tp: Tetrahymena pyriformis
TW: tap water
WO: waste oils
Symbols definition