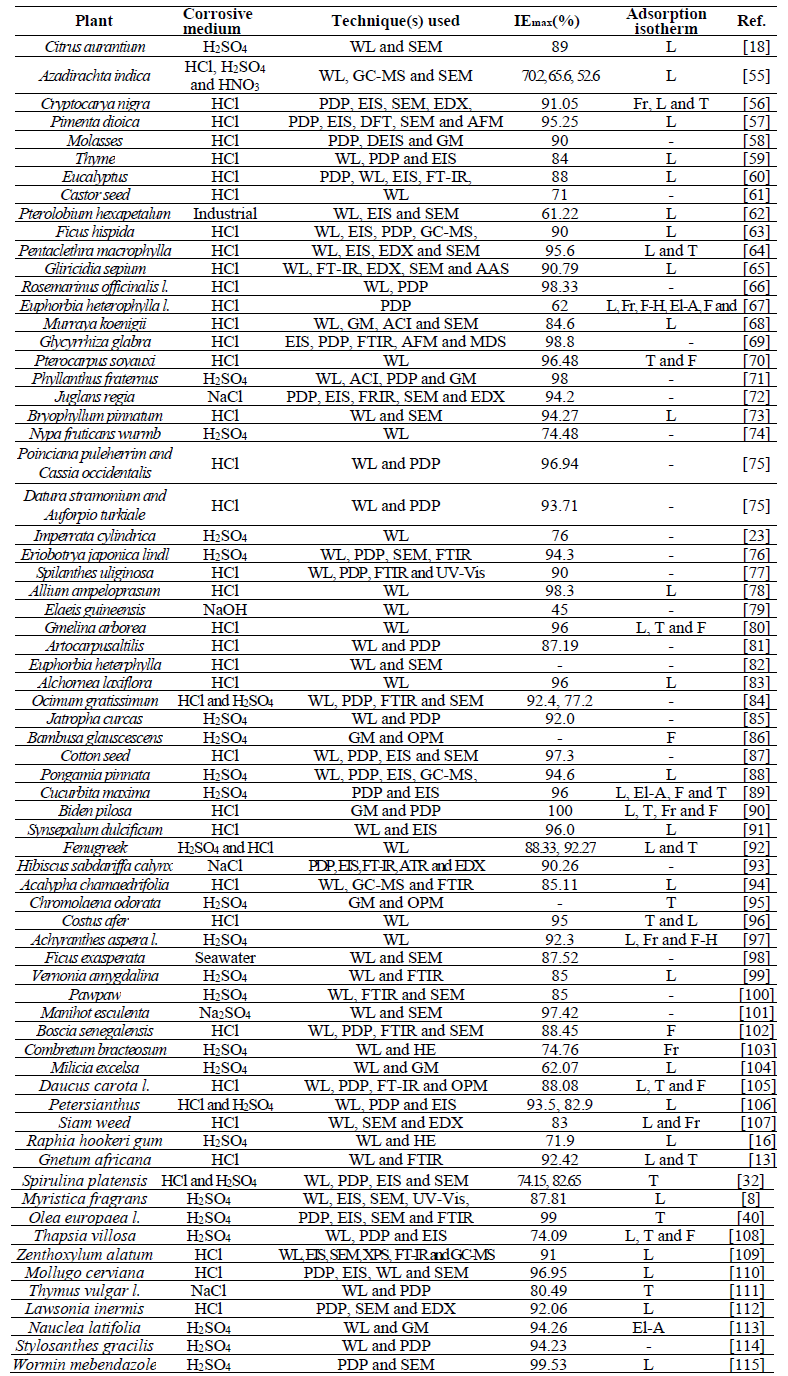
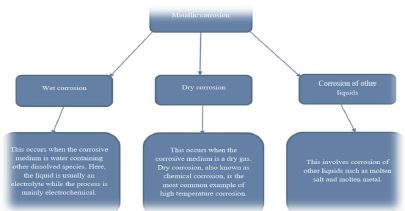
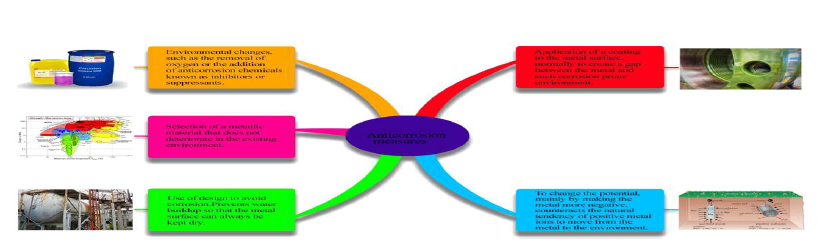
References
1. Oyejide J, Orhorhoro E, Ogie A, et al. Investigation of the Effect of Annealing on the Corrosion Resistance of Medium Carbon Steel in Sea Water. J Emerg Trends Eng Appl Sci. 2017;8(5):219-24.
[ Links ]
2. Atanda P, Olorunniwo O, Alabi O, et al. Effect of Iso-Thermal Treatment on the Corrosion Behaviour of Low Carbon Steel (Nigerian C2R grade) in a Buffered Solution Containing Chloride and Carbonate Ions. Int J Mater Chem. 2012;2(2):65-71. DOI: https://doi.org/10.5923/j.ijmc.20120202.04
[ Links ]
3. Adebayo A, Ekiti A, Stephen J, et al. Corrosion Behaviour of Heat Treated and Nickel Plated Mild Steel in Citrus Fruit: Lime and Lemon. Int J Sci Eng Technol Res. 2019;08:229-35.
[ Links ]
4. Shukla S, Ebenso E. Corrosion Inhibition, Adsorption Behavior and Thermodynamic Properties of Streptomycin on Mild Steel in Hydrochloric Acid Medium. Int J Electrochem Sci. 2011;6:3277-91.
[ Links ]
5. Ameh P, Eddy N. Theoretical and experimental studies on the corrosion inhibition potentials of 3-nitrobenzoic acid for mild steel in 0.1 M H2SO4. Cogent Chem. 2016;15(1):0-18. DOI: http://dx.doi.org/10.1080/23312009.2016.1253904
[ Links ]
6. Idouhli R, Oukhrib A, Koumya Y, et al. Inhibitory effect of Atlas cedar essential oil on the corrosion of steel in 1 M HCl. Corros Rev. 2018. DOI: https://doi.org/10.1515/corrrev-2017-0076
[ Links ]
7. Gadow H, Motawea M. Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv. 2017;7:24576-88. DOI: http://dx.doi.org/10.1039/C6RA28636D
[ Links ]
8. Haldhar R, Prasad D, Saxena A. Myristica fragrans Extract as an Eco-friendly Corrosion Inhibitor for Mild Steel in 0.5 M H2SO4 Solution. J Environ Chem Eng. 2018;(6)2:2290-2301. DOI: https://doi.org/10.1016/j.jece.2018.03.023
[ Links ]
9. Bala C, Balamurugan S, Balamurugan P, et al. Corrosion inhibition of mild steel by using banana peel extract. Int J Innov Technol Explor Eng. 2019;8(6):1372-5.
[ Links ]
10. Mark U, Aharanwa I, Igwe C. The Inhibitive Effect of Carica Papaya Leaf Extract on the Corrosion of Mild Steel in Acidic ( 1 M HCl ) Medium. Int J Eng Sci. 2019;8(3):10-20. DOI: https://doi.org/10.9790/1813-0803011020
[ Links ]
11. Ajayi O, Omotosho O, Ifepe V. Acid Failure of Mild Steel in 2 M Sulphuric Acid in the Presence of Vernonia Amygdalina. J Mater Environ Sci. 2011;2(2):186-95.
[ Links ]
12. Okewale A, Olaitan A. The Use of Rubber Leaf Extract as a Corrosion Inhibitor for Mild Steel in Acidic Solution. Int J Mater Chem. 2017;7(1):5-13. http://journal.sapub.org/ijmc
[ Links ]
13. Nnanna L, Owate I, Nwadiuko O, et al. Adsorption and Corrosion Inhibtion of Gnetum Africana Leaves Extract on Carbon Steel. Int J Mater Chem. 2013;3(1):10-6. DOI: https://doi.org/10.5923/j.ijmc.20130301.03
[ Links ]
14. Malaibari Z, Kahraman R, Rauf A. Corrosion of inhibitor treated mild steel immersed in distilled water and a simulated salt solution. Anti-Corros Methods Mater. 2013;60(5):227-33. DOI: https://doi.org/10.1108/ACMM-06-2012-1186
[ Links ]
15. Mo S, Luo H, Li N. Plant extracts as "green" corrosion inhibitors for steel in sulphuric acid. Chem Pap. 2016;70(9):1131-43. DOI: https://doi.org/10.1515/chempap-2016-0055
[ Links ]
16. Umoren S, Obot I, Obi-Egbedi N. Raphia hookeri gum as a potential eco-friendly inhibitor for mild steel in sulfuric acid. J Mater Sci. 2009;44(1):274-9. DOI: https://doi.org/10.1007/s10853-008-3045-8
[ Links ]
17. Mobin M, Rizvi M. Inhibitory effect of xanthan gum and synergistic surfactant additives for mild steel corrosion in 1 M HCl. Carbohydr Polym. 2016;136:384-93. DOI: https://doi.org/10.1016/j.carbpol.2015.09.027
[ Links ]
18. Hassan K, Khadom A, Kurshed N. Citrus aurantium leaves extracts as a sustainable corrosion inhibitor of mild steel in sulfuric acid. South African J Chem Eng. 2016;22:1-5. DOI: https://doi.org/10.1016/j.sajce.2016.07.002
[ Links ]
19. Loto R. Corrosion inhibition of mild steel in acidic medium by butyl alcohol. Res Chem Intermed. 2013;39(2):1899-910. DOI: https://doi.org/10.1007/s11164-013-1088-1
[ Links ]
20. Möller H, Boshoff E, Froneman H. The corrosion behaviour of a low carbon steel in natural and synthetic seawaters. J South African Inst Min Metall. 2006;106(8):585-92.
[ Links ]
21. Nwigwe S, Reginald Umunakwe, Moses Y, et al. The inhibition of Carica papaya leaves extract on the corrosion of cold worked and annealed mild steel in HCl and NaOH solutions using a weight loss technique. EASR. 2019;46:114-9. DOI: https://doi.org/10.14456/easr.2019.14
[ Links ]
22. Makanjuola O, Paul A, Fasakin J. Performance of Mild Steel in Nitric Acid/ Carica Papaya Leaf Extracts Corrosion System. Asian J Appl Sci. 2015;3:110-116.
[ Links ]
23. Ofuyekpone O, Akaluzia R, Edibo S. Investigating the influence of immersion time and inhibitor concentration on the inhibiting potential of Imperrata cylindrica as corrosion inhibitor of mild steel. Int J Res Eng Innov. 2017;1(6):147-52. DOI: http://www.ijrei.com
[ Links ]
24. Ndukwe A, Anyakwo C. Modelling of Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Crushed Leaves of Sida Acuta (Malvaceae). Int J Eng Sci. 2017;06(01):22-33. DOI: https://doi.org/10.9790/1813-0601032233
[ Links ]
25. Aramide F. Corrosion Inhibition of AISI/SAE Steel in a Marine Environment. Leonardo J Sci. 2009;(15):47-52.
[ Links ]
26. Debi G, Esah H, Mohammad I, et al. Effect of Vernonia Amygdalina Extract on Corrosion Inhibition of Mild Steel in Simulated Seawater. Aust J Basic Appl Sci. 2013;7(14):257-63.
[ Links ]
27. Durowaye S, Sekunowo O, Durowaye V. Inhibitive Behaviour of Methyl Red Corrosion of Mild Steel in Alkaline Medium. Amer J Mater Sci. 2014;4(2):111-7. DOI: https://doi.org/10.5923/j.materials.20140402.08
[ Links ]
28. Ugwu B, Ekwuribe S, Onukwuli O. Corrosion inhibition of papaya (pawpaw) leaf extract on mild steel in HNO3 and NaOH media. Umudike J Eng Technol. 2017;3(1):2-4.
[ Links ]
29. Abeng F, Ikpi M, Anadebe V, et al. Metolazone compound as corrosion inhibitor for API 5L X-52 steel in hydrochloric acid solution. Bull Chem Soc Ethiop. 2020;34(2):407-18. DOI: https://doi.org/10.4314/bcse.v34i2.16
[ Links ]
30. Buchweishaija J. Plants as a Source of Green Corrosion Inhibitors: The Case of Gum Exudates from Acacia Species (A. drepanolobium and A. senegal). Tanzania J Sci. 2009;(35):93-106.
[ Links ]
31. Rani B, Basu B. Green inhibitors for corrosion protection of metals and alloys: An overview. Int J Corros. 2012:1687-9325. DOI: http://doi.org/10.1155/2012/380217
[ Links ]
32. Kamal C, Sethuraman M. Spirulina platensis - A novel green inhibitor for acid corrosion of mild steel. Arab J Chem. 2012;5(2):155-61. DOI: http://doi.org/10.1016/j.arabjc.2010.08.006
[ Links ]
33. Yaro A, Khadom A, Wael R. Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alexandria Eng J. 2013;52(1):129-35. DOI: http://doi.org/10.1016/j.aej.2012.11.001
[ Links ]
34. Osuwa C, Okere C. Aspilia Africana extracts as organic corrosion inhibitor of mild steel in corrosive acidic media. IOSR J Environ Sci Toxicol Food Technol. 2013;4(5):61-5. DOI: https://doi.org/10.9790/2402-0456165
[ Links ]
35. Abiola O, James A. The effects of Aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corros Sci. 2010;52(2):661-4. DOI: http://doi.org/10.1016/j.corsci.2009.10.026
[ Links ]
36. Li X, Deng S, Fu H. Inhibition of the corrosion of steel in HCl and H2SO4 solutions by bamboo leaf extract. Corros Sci. 2012;62:163-75. DOI: http://doi.org/10.1016/j.corsci.2012.05.008
[ Links ]
37. Odejobi J, Akinbulumo A. Modeling and optimization of the inhibition efficiency of Euphorbia heterophylla extracts based corrosion inhibitor ?f mild steel corrosion in HCl media using ? response surface methodology. J Chem Technol Metall. 2018;54(1):217-32.
[ Links ]
38. Olawale O, Oyawale F, Adediran A, et al. Corrosion Inhibition of Mild Steel in Seawater using Jatropha Stem. Analele Univ Eftimie Murgu Resita Fasc Ing. 2016;23(1):228-38.
[ Links ]
39. Ofuyekpone O, Emordi N, Utu O. Effect of sulphuric acid concentration on the inhibiting action of 0.001 M adenine solution during the corrosion of AISI 304L. Adv Appl Sci Res. 2015;6(9):17-26.
[ Links ]
40. Düdükcü M, Kaplan S, Avci G. Green Approach to Corrosion Inhibition of Mild Steel in Sulphuric Acid Solution by the Extract of Olea Europaea Leaves. J Mater Environ Sci. 2020;11(1):45-56.
[ Links ]
41. Raja P, Qureshi A, Abdul A, et al. Neolamarckia cadamba alkaloids as eco-friendly corrosion inhibitors for mild steel in 1 M HCl media. Corros Sci. 2013;69:292-301. DOI: http://dx.doi.org/10.1016/j.corsci.2012.11.042
[ Links ]
42. Mathina A, Rajalakshmi R. Corrosion inhibition of mild steel in acid medium using Canna Indica as green corrosion inhibitor. Rasayan J Chem. 2016;9(1):56-66.
[ Links ]
43. Subhashini S, Rajalakshmi R, Prithiba A, et al. Corrosion mitigating effect of Cyamopsis Tetragonaloba seed extract on mild steel in acid medium. E-J Chem. 2010;7(4):1133-7. DOI: https://doi.org/10.1155/2010/457825
[ Links ]
44. Hart K, Orubite-Okorosaye K, Abodsede O. Corrosion Inhibition of Mild Steel in Simulated Seawater by Nymphae Pubscens Leaf Extracts (NLE). Int J Adv Res Chem Sci. 2017;4(12):32-40. DOI: https://doi.org/10.20431/2349-0403.0412004
[ Links ]
45. Parthipan P, Elumalai P, Narenkumar J, et al. Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. Int Biodeterior Biodegrad. 2018;132:66-73. DOI: https://doi.org/10.1016/j.ibiod.2018.05.005
[ Links ]
46. Loto R, Olowoyo O. Synergistic effect of sage and jojoba oil extracts on the corrosion inhibition of mild steel in diluted acid solution. Procedia Manufacturing. 2019;35:310-4. DOI: https://doi.org/10.1016/j.promfg.2019.05.045
[ Links ]
47. Ladan M, Basirun W, Kazi S, et al. Corrosion protection of AISI 1018 steel using Co-doped TiO2/polypyrrole nanocomposites in 3.5% NaCl solution. Mater Chem Phys. 2017;192:361-73. DOI: http://doi.org/10.1016/j.matchemphys.2017.01.085
[ Links ]
48. Miralrio A, Vazquez A. Plant Extracts as Green Corrosion Inhibitors for Different Metal Surfaces and Corrosive Media: a Review. Processes. 2020;8(8):942. DOI: https://doi.org/10.3390/pr8080942
[ Links ]
49. Kaur J, Daksh N, Saxena A. Corrosion Inhibition Applications of Natural and Eco-Friendly Corrosion Inhibitors on Steel in the Acidic Environment: an Overview. Arab J Sci Eng. 2021. DOI: https://doi.org/10.1007/s13369-021-05699-0
[ Links ]
50. Sastri V. Green corrosion inhibitors. New Jersey: John Wiley & Sons, Inc. 2011:1-310.
[ Links ]
51. Bardal E. Corrosion and Protection. London: Springer-Verlag London Limited. 2003:1-315.
[ Links ]
52. Fayomi O, Akande I, Odigie S. Economic Impact of Corrosion in Oil Sectors and Prevention: an Overview. J Phys Conf Ser. 2019;1378(2):1-8.
[ Links ]
53. Verma C, Quraishi MA. Adsorption behavior of 8,9-bis(4 (dimethyl amino)phenyl)benzo(4,5)imidazo(1,2-a)pyridine-6,7-dicarbonitrile on mild steel surface in 1 M HCl. J Assoc Arab Univ Basic Appl Sci. 2017;22:55-61. DOI: http://doi.org/10.1016/j.jaubas.2016.01.003
[ Links ]
54. Chigondo M, Chigondo F. Recent Natural Corrosion Inhibitors for Mild Steel: an Overview. J Chem. 2016;2016:1-7.
[ Links ]
55. Peter A, Sharma S. Use of Azadirachta indica (AZI) as green corrosion inhibitor against mild steel in acidic medium: anti-corrosive efficacy and adsorptive behaviour. Int J Corros Scale Inhib. 2017;6(2):112-31.
[ Links ]
56. Faiz M, Zahari A, Awang K, et al. Corrosion inhibition on mild steel in 1 M HCl solution by Cryptocarya nigra extracts and three of its constituents (alkaloids). RSC Adv. 2020;10(11):6547-62.
[ Links ]
57. Anupama K, Ramya K, Shainy K, et al. Adsorption and electrochemical studies of Pimenta dioica leaf extracts as corrosion inhibitor for mild steel in hydrochloric acid. Mater Chem Phys. 2015;167:28-41. DOI: https://doi.org/10.1016/j.matchemphys.2015.09.013
[ Links ]
58. Slepski P, Gerengi H, Jazdzewska A, et al. Simultaneous impedance and volumetric studies and additionally potentiodynamic polarization measurements of molasses as a carbon steel corrosion inhibitor in 1 M hydrochloric acid solution. Constr Build Mater. 2014;52:482-7. DOI: https://doi.org/10.1016/j.conbuildmat.2013.11.059
[ Links ]
59. Ibrahim T, Alayan H, Mowaqet Y. The effect of Thyme leaves extract on corrosion of mild steel in HCl. Prog Org Coatings. 2012;75(4):456-62. DOI: http://doi.org/10.1016/j.porgcoat.2012.06.009
[ Links ]
60. Dehghani A, Bahlakeh G, Ramezanzadeh B. Green Eucalyptus leaf extract: A potent source of bio-active corrosion inhibitors for mild steel. Bioelectrochemistry. 2019;130:107339. DOI: https://doi.org/10.1016/j.bioelechem.2019.107339
[ Links ]
61. Srivastava K, Srivastava P. Studies-on plant materials as corrosion inhibitors. Br Corros J. 1981;16(4):221-3.
[ Links ]
62. Pradeep K, Mohana K. Phytochemical screening and corrosion inhibitive behavior of Pterolobium hexapetalum and Celosia argentea plant extracts on mild steel in industrial water medium. Egypt J Pet. 2014;23(2):201-11. DOI: http://doi.org/10.1016/j.ejpe.2014.05.007
[ Links ]
63. Muthukrishnan P, Prakash P, Jeyaprabha B, et al. Stigmasterol extracted from Ficus hispida leaves as a green inhibitor for the mild steel corrosion in 1 M HCl solution. Arab J Chem. 2019;12(8):3345-56. DOI: http://doi.org/10.1016/j.arabjc.2015.09.005
[ Links ]
64. Nnanna L, Owate I, Oguzie E. Inhibition of Mild Steel Corrosion in HCl Solution by Pentaclethra macrophylla Bentham Extract. Int J Mater Eng. 2014;4(5):171-9.
[ Links ]
65. Okoronkwo A, Olusegun S, Olaniran O. Acid extract of Gliricidia sepium leaves as green corrosion inhibitor for mild steel in HCl solutions. African Corros J. 2015;1(1):1-5.
[ Links ]
66. Rahman M, Gul S, Umair M, et al. Anticorrosive Activity of Rosemarinus Officinalis Leaves Extract Against Mild Steel in Dilute Hydrochloric Acid. Int J Eng. 2016;3(03):38-43. http://www.ijirae.com/volumes/Vol3/iss3/08.MRAE10085.pdf
[ Links ]
67. Akinbulumo O, Odejobi O, Odekanle E. Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Results Mater. 2020;5:100074. DOI: https://doi.org/10.1016/j.rinma.2020.100074
[ Links ]
68. Sharmila A, Prema A, Sahayaraj P. Influence of Murraya koenigii (curry leaves) extract on the corrosion inhibition of carbon steel in HCL solution. Rasayan J Chem. 2010;3(1):74-81.
[ Links ]
69. Alibakhshi E, Ramezanzadeh M, Haddadi S, et al. Persian Liquorice extract as a highly efficient sustainable corrosion inhibitor for mild steel in sodium chloride solution. J Clean Prod. 2019;210:660-72. DOI: https://doi.org/10.1016/j.jclepro.2018.11.053
[ Links ]
70. Iloamaeke I, Onuegbu T. Corrosion Inhibition Of Mild Steel by Pterocarpus Soyauxi Leaves Extract in HCl Medium?. Int J Plant Anim Environ Sci. 2012;2(3):22-8. http://ijpaes.com/admin/php/uploads/192_pdf.pdf
[ Links ]
71. Patel N, Hrdlicka J, Beranek P, et al. Extract of Phyllanthus fraternus leaves as corrosion inhibitor for mild steel in H2SO4 solutions. Int J Electrochem Sci. 2014;9(6):2805-15.
[ Links ]
72. Haddadi S, Alibakhshi E, Bahlakeh G, et al. A detailed atomic level computational and electrochemical exploration of the Juglans regia green fruit shell extract as a sustainable and highly efficient green corrosion inhibitor for mild steel in 3.5 wt% NaCl solution. J Mol Liq. 2019;284:682-99. DOI: https://doi.org/10.1016/j.molliq.2019.04.045
[ Links ]
73. Kumar D, Khan F. Corrosion Inhibition of Mild Steel by Extract of Bryophyllum Pinnatum Leaves in Acidic Solution. Chem Mater Res. 2015;7(5):69-77. www.iiste.org
[ Links ]
74. Michael N. The Corrosion Inhibition of Mild Steel in Sulphuric Acid Solution by Flavonoid (Catechin) Separated from Nypa Fruticans Wurmb Leaves Extract. Sci J Chem. 2014;2(4):27.
[ Links ]
75. Zucchi F. Plant extracts as corrosion inhibitors of mild steel in HCl solutions. Surf Technol. 1985;24:391-9.
[ Links ]
76. Zheng X, Gong M, Li Q, et al. Corrosion inhibition of mild steel in sulfuric acid solution by loquat (Eriobotrya Japonica Lindl) leaves extract. Sci Rep. 2018;8(9140):1-15. DOI: http://doi.org/10.1038/s41598-018-27257-9
[ Links ]
77. Durodola S, Adekunle A, Olasunkanmi L, et al. Inhibition of Mild Steel Corrosion in Acidic Medium by Extract of Spilanthes uliginosa Leaves. Electroanalysis. 2020;32(12): 2693-2702. DOI: http://doi.org/10.1002/elan.202060227
[ Links ]
78. Hussein H, Sultan A, Intisar A, et al. Mechanical Properties of Carbon Steel Using of Allium Ampeloprasum Extract as Corrosion Inhibitor. Int J Mech Eng Technol. 2015;6(1):34-41.
[ Links ]
79. Abhulimen E. An investigation on the optimal concentration of oil palm (Elaeis guineensis) leaves extract as corrosion inhibitor of carbon steel in deaerated saline solution. MOJ Appl Bionics Biomech. 2018;60(2):109-13.
[ Links ]
80. Nnanna L, Uchendu K, Nwosu F, et al. Gmelina Arborea Bark Extracts as a Corrosion Inhibitor for Mild Steel in an Acidic Environment. Int J Mater Chem. 2014;4(2):34-9. DOI: https://doi.org/10.5923/j.ijmc.20140402.03
[ Links ]
81. Oruene D, Isaac O, Nkoi B. The Inhibition Effect of Breadfruit Leaf Extract on Mild Steel Corrosion. J Newviews Eng Technol. 2021;3(1):34-43.
[ Links ]
82. Aliyu A, Salisu L, Akilu A, et al. Mild Steel Corrosion Inhibition by Euphorbia heterphylla Extract in 0.5 M Hydrochloric Acid Solution. J Mater Environ Sci. 2021;12(3):431-41.
[ Links ]
83. Olasehinde E, Ogunjobi J. Investigation of the Inhibitive Properties of Alchornea laxiflora Leaves on the Corrosion of Mild Steel in HCl: Thermodynamics and Kinetic Study. J Amer Sci. 2015;11(1s):32-9.
[ Links ]
84. Chinonso A, Chidiebere A, Ibe F, et al. Protecting Mild Steel from Acid Corrosion Using Extract from Ocimum gratissimum Leaves. Int Lett Chem Phys Astron. 2017;73:9-21. DOI: https://doi.org/10.18052/www.scipress.com/ILCPA.73.9
[ Links ]
85. Gupta D, Das A, Neupane S, et al. Study of Jatropha Curcas Extract as a Corrosion Inhibitor in Acidic Medium on Mild Steel by Weight Loss and Potentiodynamic Methods. J Nepal Chem Soc. 2020;41(1):87-93.
[ Links ]
86. Omotosho O, Ajayi O, Fayomi O, et al. Assessing the deterioration behaviour of mild steel in 2 M sulphuric acid using Bambusa glauscescens. Int J Appl Eng Res. 2011;2(2):406-18.
[ Links ]
87. Hernandes I, Cunha D, Araujo C, et al. Application of an Aqueous Extract of Cotton Seed as a Corrosion Inhibitor for Mild Steel in HCl Media. Mater Res. 2021;24(1):1-10.
[ Links ]
88. Bhuvaneswari T, Vasantha V, Jeyaprabha C. Pongamia Pinnata as a Green Corrosion Inhibitor for Mild Steel in 1 N Sulfuric Acid Medium. Silicon. 2018; 10(1):1-14. DOI: https://doi.org/10.1007/s12633-017-9673-3
[ Links ]
89. Anbarasi K, Vasudha V. Influence of eco friendly plant material (Cucurbita maxima) on mild steel corrosion. Anti-Corros Methods Mater. 2016:1-13. DOI: https://doi.org/10.1108/ACMM-09-2016-1709
[ Links ]
90. Ajayi S, Ademosun O, Okoro ER, et al. Corrosion Inhibitory Properties of Biden Pilosa Plant Extract on Mild Steel in Acidic Media. J Phys: Conference Series. 2019:1-10. DOI: https://doi.org/10.1088/1742-6596/1378/4/042005
[ Links ]
91. Nnenna W, Adeola S, Msenhemba M, et al. Synsepalum Dulcificum Leaves Extract as Green Inhibitor for Mild Steel Corrosion in Hydrochloric Acid. Chem Search J. 2021;12(1):47-54.
[ Links ]
92. Noor E. Temperature Effects on the Corrosion Inhibition of Mild Steel in Acidic Solutions by Aqueous Extract of Fenugreek Leaves. Int J Electrochem Sci. 2007;2:996-1017.
[ Links ]
93. Afandi M, Saud S, Hamzah E. Green-based corrosion protection for mild steel in 3.5% NaCl and distilled water medias: Jatropha curcas and Roselle extracts. J Met Mater Miner. 2020;30(2):91. DOI: https://doi.org/104.10.14456/jmmm.2020.25
[ Links ]
94. Ebuka D, Stephen A, Abechi E. Corrosion inhibition studies of mild steel using Acalypha chamaedrifolia leaves extract in hydrochloric acid medium. SN Appl Sci. 2019;1(1089):1-11. DOI: https://doi.org/10.1007/s42452-019-1138-4
[ Links ]
95. Omotosho O, Ajayi O, Ajanaku K, et al. Environment Induced Failure of Mild Steel in 2 M Sulphuric Acid Using Chromolaena Odorata. J Mater Environ Sci. 2012;3(1):66-75.
[ Links ]
96. Lebe N, Wisdom J, Tochukwu E, et al. Inhibitive Effect of Costus Afer Extracts on Mild Steel Corrosion in Acidic Medium. Int J Eng Technol. 2016;7:60-7. DOI: https://doi.org/10.18052/www.scipress.com/IJET.7.60
[ Links ]
97. Nwosu F, Nnanna L, Okeoma K. Corrosion inhibition for mild steel in 0.5 M H2SO4 solution using Achyranthes aspera leaf extract. African J Pure Appl Chem. 2013;7(2):56-60. DOI: https://doi.org/10.5897/AJPAC2012.0073
[ Links ]
98. Oyewole O, Aondoakaa E, Abayomi T, et al. Characterization and optimization study of Ficus exasperata extract as corrosion inhibitor for mild steel in seawater. World Sci News. 2021;151:78-94.
[ Links ]
99. Achebe C, Ilogebe A, Chukwuneke J, et al. The Effects of Inhibition on Corrosion of Mild Steel in H2SO4 Using Ethanol Extract of Vernonia Amygdalina. Int J Eng Sci. 2015;4(8):28-36.
[ Links ]
100. Chukwueze G, Asadu C, Onu C, et al. Evaluation of the Corrosion Inhibitive Properties of Three Different Leave Extracts on Mild Steel Iron in Sulphuric Acid Solution. J Eng Res Reports. 2020;12(3):6-17. DOI: https://doi.org/10.9734/JERR/2020/v12i317080
[ Links ]
101. Madawa N, Tuaweri T, Mebine P, et al. Corrosion Inhibition of Mild Steel C1026 Pipeline in Na2SO4 Using Green Inhibitors. Int J Eng Sci Invent. 2021;10(1):15-22. DOI: https://doi.org/10.35629/6734-1001011522
[ Links ]
102. Awe F, Idris S, Abdulwahab M, et al. Theoretical and experimental inhibitive properties of mild steel in HCl by ethanolic extract of Boscia senegalensis. Cogent Chem. 2015;1:1-14. DOI: https://doi.org/10.1080/23312009.2015.1112676
[ Links ]
103. Okafor P, Uwah I, Ekerenam O, et al. Combretum bracteosum extracts as eco-friendly corrosion inhibitor for mild steel in acidic medium. Pigment Resin Technol. 2009;38(4):236-41. DOI: https://doi.org/10.1108/03699420910973323
[ Links ]
104. Ishmael V, Ajiwe E, Ejike C. Corrosion Inhibition of Mild Steel in Sulphuric Acid by Methanol Leaf Extracts of Milicia Excelsa. Open Sci J Analyt Chem. 2019;4(2):13-9.
[ Links ]
105. Saeed M, Saleem M, Niyazi A, et al. Carrot (Daucus Carota) Peels Extract as an Herbal Corrosion Inhibitor for Mild Steel in 1 M HCl Solution. Mod Appl Sci. 2020;14(2):97-112. DOI: https://doi.org/10.5539/mas.v14n2p97
[ Links ]
106. Akalezi C, Enenebaku C, Oguzie E. Inhibition of acid corrosion of mild steel by biomass extract from the Petersianthus macrocarpus plant. J Mater Environ Sci. 2013;4(2):217-26.
[ Links ]
107. Olusegun S, Okoronkwo E, Okotete A. Gravimetric and electrochemical studies of corrosion inhibition potential of acid and ethanol extract of siam weed on mild steel. Leonardo J Sci. 2016;(29):25-42.
[ Links ]
108. Kalla A, Benahmed M, Djeddi N, et al. Corrosion inhibition of carbon steel in 1 M H2SO4 solution by Thapsia villosa extracts. Int J Ind Chem. 2016;7(4):419-29. DOI: https://doi.org/10.1007/s40090-016-0094-8
[ Links ]
109. Chauhan L, Gunasekaran G. Corrosion inhibition of mild steel by plant extract in diluted HCl medium. Corros Sci. 2007;49(3):1143-61.
[ Links ]
110. Arockiasamy P, Thenmozhi G, Franco M, et al. Evaluation of Corrosion Inhibition of Mild Steel in 1 M Hydrochloric Acid Solution by Mollugo Cerviana. Int J Corros. 2014;2014:1-7. DOI: https://doi.org/10.1155/2014/679192
[ Links ]
111. Premkumar P, Kannan K, Natesan M. Thyme extract of Thymus vulgar leaves as volatile corrosion inhibitor for mild steel in NaCl environment. Asian J Chem. 2008;20(1):445-51.
[ Links ]
112. Ostovari A, Hoseinieh SM, Peikari M, et al. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (lawsone, gallic acid, a-d-glucose and tannic acid). Corros Sci. 2009;51(9):1935-49.
[ Links ]
113. Uwah I, Okafor P, Ebiekpe V. Inhibitive action of ethanol extracts from Nauclea latifolia on the corrosion of mild steel in H2SO4 solutions and their adsorption characteristics. Arab J Chem. 2013;6(3):285-93. DOI: http://doi.org/10.1016/j.arabjc.2010.10.008
[ Links ]
114. Dickson O, Goodluck O, Onyekpe B, et al. Effect of inhibitor concentration on the potency of Stylosanthes gracilis extract as corrosion inhibitor for AISI 304l in sulphuric acid solution. Sci Afric. 2021;11(e00714):1-4. DOI: https://doi.org/10.1016/j.sciaf.2021.e00714
[ Links ]
115. Edoziuno F, Adediran A, Odoni B, et al. Influence of Wormin Mebendazole on the Corrosion of Mild Steel in 1.0 M Sulphuric Acid. Results Eng. 2020;9:100192. DOI: https://doi.org/10.1016/j.rineng.2020.100192
[ Links ]
116. Ameer M, Fekry A. Corrosion inhibition of mild steel by natural product compound. Prog Org Coatings. 2011;71(4):343-9. DOI: http://doi.org/10.1016/j.porgcoat.2011.04.001
[ Links ]
117. Chaudhari H, Vashi R. The study of henna leaves extract as green corrosion inhibitor for mild steel in acetic acid. J Fundam Appl Sci. 2016;8(2):280. DOI: https://doi.org/10.4314/jfas.v8i2.8
[ Links ]
118. Noyel S, Prasad R, Manivannan R. Psidium guajava leaf extract as green corrosion inhibitor for mild steel in phosphoric acid. Int J Electrochem Sci. 2015;10(3):2220-38.
[ Links ]
119. Li X, Deng S, Li N, et al. Inhibition effect of bamboo leaves extract on cold rolled steel in Cl3CCOOH solution. J Mater Res Technol. 2017;6(2):158-70. DOI: http://doi.org/10.1016/j.jmrt.2016.09.002
[ Links ]
120. Singh M, Gupta A. Corrosion behaviour of mild steel in formic acid solutions. Mater Chem Phys. 1996;46(1):15-22. DOI: https://doi.org/10.1016/0254-0584(96)80124-6
[ Links ]
121. Yadav M, Gope L, Kumari N, et al. Corrosion inhibition performance of pyranopyrazole derivatives for mild steel in HCl solution: gravimetric, electrochemical and DFT studies. J Mol Liq. 2016;216:78-86. DOI: http://doi.org/10.1016/j.molliq.2015.12.106
[ Links ]
122. Satapathy A, Gunasekaran G, Sahoo S, et al. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros Sci. 2009;51(12):2848-56. DOI: https://doi.org/10.1016/j.corsci.2009.08.016
[ Links ]
123. Alibakhshi E, Ramezanzadeh M, Bahlakeh G, et al. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J Mol Liq. 2018;255:185-98. DOI: https://doi.org/10.1016/j.molliq.2018.01.144
[ Links ]