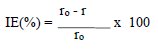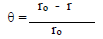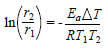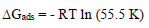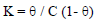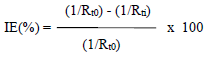Introduction
The study of steel corrosion in acidic solutions is an important field of industrial research. Steel corrosion inhibition in acidic solutions, by different types of organic compounds, has been extensively studied (1-5. Surfactants have been used as corrosion inhibitors by some authors (6-8. They are chemical compounds with amphiphilic molecules, which contain hydrophobic (non-polar) tails and hydrophilic (polar) heads 9.
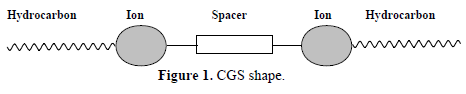
Recently, the new generation of surfactants called CGS has also been used as corrosion inhibitors in acidic solutions (10,11. It was found that they are good corrosion inhibitors, due to their more efficient surface properties, with low values of CMC and surface energy. These compounds have marked IE(%) near their CMC values. CGS molecules consist of two typical surfactant monomers that are covalently linked together by either a rigid or flexible spacer group, i.e., containing two hydrophilic and hydrophobic groups (sometimes three, if the spacer itself includes a hydrophobic chain) 12,13. In 1991, the name CGS was assigned to a group of amphiphiles possessing, in sequence, a long hydrocarbon chain, an ionic group, a spacer, a second ionic group and another hydrocarbon tail (Fig. 1) (8.
Short and flexible spacers are preferred, since a “hair pin” turn can be aligned, and two chains of a molecule will lead to micelle formation (6,10,11. These surfactants show superior performance than that of conventional surfactants that are made up of one polar and one hydrophilic moieties. They have much lower CMC and better wetting properties (14,15.
In the present research work, the corrosion inhibition action of four CGS (PPAB, HPAB, DDPAB and HDPAB) was investigated on MS in HCl solutions, by gravimetric measurements (WL), PDP and EIS studies.
Experimental procedures
Gravimetric measurements
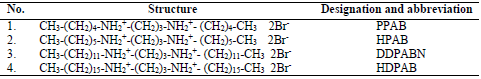
The principle of this method is based on WL measurement of the MS sample after its immersion in HCl, without and with CGS. HCl (Merck) of AR grade and double distilled water were used to prepare the solutions. CGS were prepared with dibromopropane and the corresponding alkyl amine in absolute C2H6O, under reflux, for 24 h. After C2H6O rotatory evaporation, a product was obtained. The resulting product was recrystallized in pure C3H6O and C3H6O-C2H6O mixtures (8). The compounds names and molecular structures are given in Table 1.
WL experiments were carried out in 1 N HCl, using cold rolled MS samples (2 x 2 x 0.25 cm), with the composition (wt%) of 0.14% C, 0.35% Mn, 0.17% Si, 0.025% S, 0.03% P and balance Fe, as the per standard method 16. For the experiment, coupons were cold-cut from a MS sheet. They were polished using SiC emery papers, washed with C2H6O, then degreased with C3H6O, for 1 min, followed by rinsing with C2H6O and deionized water. Finally, they were dried at air and used.
PDP studies
For PDP studies, MS strips embedded in araldite (a fixed material), with an exposed area of 1.0 cm2, were used as WE. The experiments were done at a constant T of 30+2 ºC, as per ASTM G3-74 and G5-87. PDP studies were carried out using an EG&G PARC potentiostat/galvanostat (model 173), a universal programmer (model 175) and a X-Y recorder (model RE 0089). MS was used as WE, a Pt foil was used as AE and SC was used as RE.
EIS studies
Impedance measurements were performed for MS in 1 N HCl, at room T, without and with 50 and 250 ppm HDPAB, at Ecorr with the AC voltage amplitude of 5 mV, in the frequency range from 5 to 100 Hz. A time interval of few min was given, for the OCP to reach a steady value. All the measurements were carried out with an EG&G PAR (model 5301A) lock-in-amplifier. Only HDPAB was selected for EIS studies.
Results and discussion
Gravimetric measurements
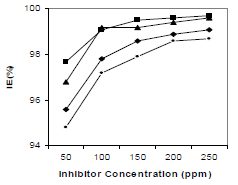
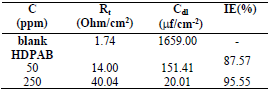
IE% and CR values obtained by gravimetric measurements, using the WL method, with different inhibitor C, at 30 ºC, for 3 h, are summarized in Table 2 and shown in Fig. 2.
According to data in Table 2, MS CR values decreased with higher inhibitors C. The maximum IE(%) and minimum CR of each CGS were achieved at 250 ppm, in a 1 N HCl solution. Further increase in C did not cause any appreciable change in the inhibitors performance. IE(%) and ( value for each inhibitor C were calculated using the following equation:
where ro and r are CR without and with inhibitors, respectively.
Table 2 Corrosion parameters for MS in 1 N HCl, without and with different C of CGS, at 30 ºC, for 3 h, from WL measurements.
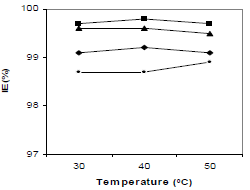
T influence on CGS IE(%) at optimum C is shown in Fig. 3. IE(%) for all the compounds in 1 N HCl did not show any significant change with the increase in T from 30 to 50 ºC, indicating that the inhibitive film formed on the metal surface kept its protective nature at higher T. The four compounds IE(%) variation with IT is shown in Fig. 4. There was no significant change in the four CGS IE(%), with an increase in IT from 3 to 12 h (Fig. 4), which shows that they protected MS from corrosion in 1 N HCl, during longer exposures.
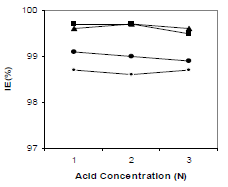
HCl C effect on 250 ppm CGS IE(%), for 3 h, is shown in Fig. 5. It was found that, with an increase in HCl C, there was no significant change in the CGS IE(%). It is clear from Fig. 5 that all tested inhibitors had good performance in 1, 2 and 3 N HCl.
Ea values were calculated using Arrhenius equation (17,18.
where r1 and r2 are CR at T1 and T2, respectively: (T = T2 - T1.
(Gads at different T was calculated from the following equation (19:
and the equilibrium constant (K) was given by the following equation:
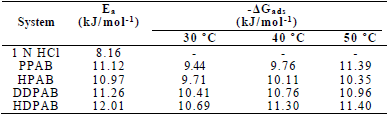
Ea values in blank 1 N HCl and with PPAB, HPAB, DDPAB and HDPAB are shown in Table 3.
It was found that Ea was higher in CGS presence, which indicates physical adsorption of these compounds onto the MS surface 19. (Gads low and negative values indicate the inhibitors spontaneous adsorption onto the metal surface 20. (Gads negative values also suggest a strong interaction of CGS molecules with the metal surface 7.
Adsorption isotherm
CGS adsorption behavior onto the metal surface must be known, so that the corrosion inhibition mechanism is understood (21. ( values for different inhibitors C were evaluated using CR values obtained from the WL method, and they were graphically tested by fitting them to various isotherms. A plot of log (( /1- () against log C, for different CGS C, shows a straight line, indicating that adsorption followed Langmuir’s isotherm (Fig. 6).
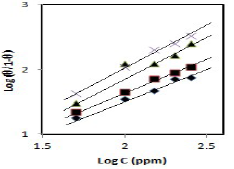
Figure 6 Langmuir’s isotherm plots for (♦) PPAB, (■) HPAB, (▲) DDPAB and (×) HDPAB adsorption onto the MS surface in 1 N HCl.
PDP studies
Cathodic and anodic polarization curves for MS in 1 N HCl with and without inhibitors, with optimum C, at 30+2 ºC, are shown in Fig. 7.
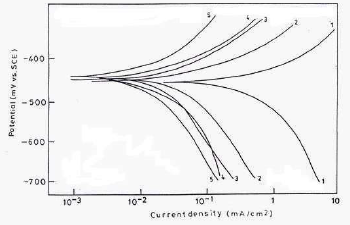
Figure 7 PDP curves for MS in 1 N HCl: (1) blank; and with 250 ppm CGS (2) PPAB; (3) HPAB; (4) DDPAB; and (5) HDPAB.
The various electrochemical parameters calculated from Tafel plots are given in Table 4.
Table 4 Electrochemical polarization parameters for MS corrosion in 1 N HCl, with optimum C of various CGS, at 30+2oC.
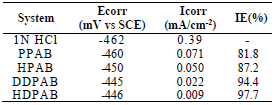
Lower Icorr values in CGS presence did not cause significant changes in Ecorr, which suggests that the compounds are mixed type inhibitors, and that they were adsorbed onto the MS surface, thereby blocking the corrosion reaction (18.
EIS
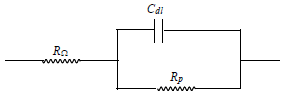
The electrical equivalent circuit for the system (Scheme 1) may be given as:
Impedance diagrams, obtained for the frequency range from 5 Hz to 100 kHz, at Ecorr, for MS in 1 N HCl, are shown in Figs. 8(a) and (b).
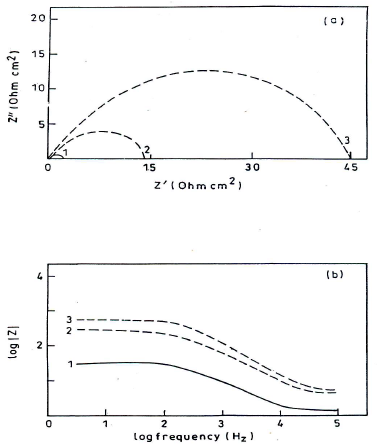
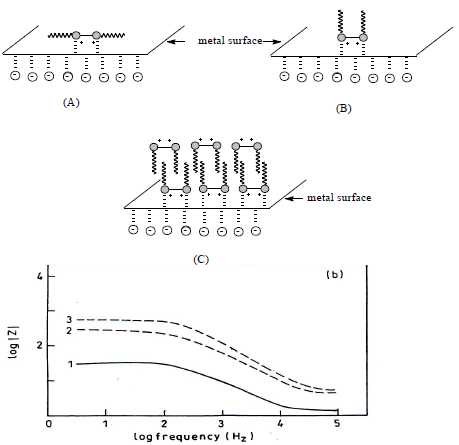
Figure 8 (a) Nyquist plot and (b) Bode plot for MS in 1 N HCl: 1. blank; 2. 50 ppm; and 3. 250 ppm HDPAB.
The impedance diagrams are not perfect semicircles, and this difference has been attributed to frequency dispersion (22. Rt and Cdl values can be evaluated using Nyquist and Bode plots (23. IE(%) was calculated using the equation:
where Rt0 and Rti are Rt without and with inhibitor, respectively. Rt values increased with higher inhibitor C and this, in turn, led to an enhanced IE(%) (Table 5). Cdl values in HDPAB presence decreased, suggesting that the inhibition was due to CGS adsorption onto the MS surface 24.
Inhibition mechanism
MS corrosion inhibition in 1 N HCl by CGS can be explained by the adsorption process. CGS molecules adsorption onto the metal surface may be due to: electrostatic attraction between charged CGS molecules and MS; interaction of unshared electron pairs in the former with the latter, or a combination of both. CGS adsorption isotherm reflected three different adsorption modes (Fig. 9).
CGS adsorption took place as follows (10: at low C, it seems that the adsorption occurred through horizontal binding to the hydrophobic region (A). This adsorption was favored by an electrostatic interaction between the two ammonium groups (N+) and cathodic sites, in the one hand, and Cl- on the metallic surface, on the other hand; when CGS C increased, a perpendicular adsorption took place, due to an inter-hydrophobic chain interaction (B); at higher inhibitor C, an efficiency plateau appeared. As EIS showed, there was one capacitive loop at high frequencies, which is attributed to the formation of a bimolecular layer on the metal surface.
The difference in PPAB, HPAB, DDPAB and HDPAB IE(%) can be explained on the basis of the number of carbon atoms in the alkyl chain length. IE(%) increased with a higher number of carbon atoms in the chain length (8. In the case of longer alkyl chain CGS, the increased hydrophobicity was mainly responsible for the more pronounced micellar growth and decreased CMC values 25.
Conclusions
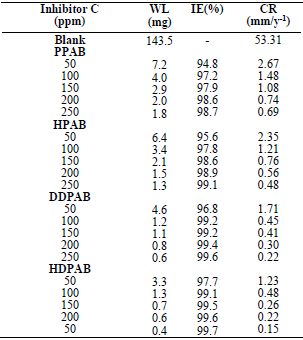
From the study of PPAB, HPAB, DDPAB and HDPAB corrosion inhibitors, the following conclusions are given:
All the tested CGS showed good performance, as inhibitors of MS corrosion in a HCl solution.
All the examined CGS acted as mixed inhibitors in a HCl solution.
The adsorption of all the four compounds onto the MS surface in the HCl solution obeyed Langmuir’s adsorption isotherm.
Acknowledgements
The author would like to acknowledge the support of Engineering Department, UTAS-Salalah-Oman
Author’s tasks
Hariom K. Sharma: conceived the original idea of the analysis and research paper; collected the data; performed the experimental work; inserted data or analysis tools; analyzed data obtained by the experiments; wrote the paper.
Abbreviations
AC: alternating current
AE: auxiliary electrode
AR: analytical reagent
C2H6O: ethanol
C3H6O: acetone
C: concentration
Cdl: double layer capacitance
CGS: gemini surfactants
CMC: critical micelle concentration
CR: corrosion rate
DDPAB: N-dodecane-diyl-1,3-propane-bis-ammonium bromide
Ea: activation energy
Ecorr: corrosion potential
EIS: electrochemical impedance spectroscope
HCl: hydrochloric acid
HDPAB: N-hexadecane-diyl-1,3-propane-bis-ammonium bromide
HPAB: N-hexane-diyl-1,3-propane-bis-ammonium bromide
Icorr: corrosion current density
IT: immersion time
IE(%): inhibition efficiency
MS: mild steel
OCP: open circuit potential
PDP: potentiodynamic polarization
PPAB: N-pentane-diyl-1,3-propane-bis-ammonium bromide
RE: reference electrode
Rt: charge-transfer resistance
SC: saturated calomel
SiC: silicon carbide
T: temperature
WE: working electrode
WL: weight loss