Serviços Personalizados
Journal
Artigo
Indicadores
Links relacionados
Compartilhar
Medicina Interna
versão impressa ISSN 0872-671X
Medicina Interna vol.26 no.1 Lisboa mar. 2019
https://doi.org/10.24950/rspmi/original/115/1/2019
ARTIGOS ORIGINAIS / ORIGINAL ARTICLES
Proton Pump Inhibitors Use for Stress Ulcer Prophylaxis in Non-Critically Ill Patients
Inibidores da Bomba de Protões na Profilaxia da Úlcera de Stress em Doentes Não Críticos
Ana C. Isabel Chora Sousa 1
 https://orcid.org/0000-0002-5182-6019
https://orcid.org/0000-0002-5182-6019
Margarida Maria Maia Leonardo Pimenta Jacinto1
 https://orcid.org/0000-0003-1327-5809
https://orcid.org/0000-0003-1327-5809
Vitória Isabel Duarte Pires2
 https://orcid.org/0000-0003-0119-7565
https://orcid.org/0000-0003-0119-7565
Tiago Tribolet de Abreu1
 https://orcid.org/0000-0001-9013-1095
https://orcid.org/0000-0001-9013-1095
1Serviço de Medicina Interna, Hospital do Espírito Santo de Évora, Évora, Portugal.
2Departamento de Endocrinologia, Hospital das Forças Armadas, Lisboa, Portugal.
ABSTRACT
Introduction: The use of proton pump inhibitors (PPIs) has a well-established role in the prophylaxis of gastrointestinal bleeding in critically care patients; however, its use in non-intensive care patients is controversial. The authors proposed to evaluate the prescription of PPIs as gastrointestinal bleeding prophylaxis during hospitalization of non-critically ill patients, including the indication and costs.
Material and Methods: A prospective, observational, cross-sectional study. All patients admitted to our medical department during a 31 day period were included. At the time of admission, a gastrointestinal bleeding risk score was completed by the attending physician. A score equal or greater than 10 points identified appropriateness of PPIs for prophylactic therapy. Costs with PPI use were collected on the study period.
Results: During the study period, 115 patients were admitted, of which 99 were included in the study (54.5% women, mean age 76.2 years). Of the gastrointestinal bleeding risk factors assessed: 28.3% acute kidney injury, 10.1% liver disease, 20.2% sepsis, 40.4% prophylactic anticoagulation, and 30.3% coagulopathy. According to the score used, 67.7% of the patients were in low or low-medium risk and 32.4% in high-medium or high risk. During hospitalization, 59.6% of the patients received PPIs, 45.8% of which inappropriately according to the risk score. The total cost of PPIs use was 101.9, with an inappropriate spending of 46.6 during a 31 day period in our Internal Medicine department alone.
Conclusion: PPIs use was prevalent in non-critically ill patients (59.6%), of which 45.8% were inappropriate, representing a problem associated with iatrogenic risk and economic impact.
Keywords: Gastrointestinal Hemorrhage/prevention & control; Peptic Ulcer/prevention & control; Proton Pump Inhibitors
RESUMO
Introdução: A utilização de inibidores da bomba de protões (IBPs) tem um papel bem estabelecido na profilaxia da hemorragia gastrointestinal no doente crítico; contudo, a sua utilização em doentes não-críticos é controversa. Os autores propuseram-se a avaliar a prescrição de IBPs em doentes num Serviço de Medicina Interna, incluindo a sua indicação e os custos associados.
Material e Métodos: : Estudo prospectivo, observacional, transversal. Foram incluídos todos os doentes admitidos no nosso Serviço de Medicina Interna durante 31 dias. Na admissão foi preenchido pelo médico assistente uma escala de risco para hemorragia gastrointestinal. A pontuação igual ou superior 10 pontos afirmava a indicação para profilaxia com IBPs. Os custos associados ao uso de IBPs foram avaliados no período do estudo.
Resultados: Foram admitidos 115 doentes, dos quais 99 doentes foram incluídos no estudo (54,5% mulheres, idade média 76,2 anos). Factores de risco: 28,3% lesão renal aguda, 10,1% patologia hepática, 20,2% sépsis, 40,4% anticoagulação profiláctica e 30,3% coagulopatia. De acordo com a escala de risco, 67,7% encontrava-se nos grupos baixo risco e 32,4% nos grupos alto risco. Verificámos que 59,6% dos doentes realizaram terapêutica com IBPs; destes, 45,8% de acordo com a escala de risco utilizada não apresentavam indicação para tal. O gasto em IBPs durante o estudo foi de 101,9, portanto o valor estimado de gastos inapropriados foi de 46,6 durante o estudo.
Conclusão: A utilização de IBPs foi elevada no nosso departamento de medicina interna (59,6%). Destes doentes a utilização de IBPs foi inapropriada em 45,8%, o que representa um problema actual com risco iatrogénico e impacto económico.
Palavras-chave: Hemorragia Gastrointestinal/prevenção e controlo; Inibidores da Bomba de Protões; Úlcera Péptica/ prevenção e controlo
Introduction
The use of proton pump inhibitors (PPIs) for the prevention of stress ulcers has been well-defined in critical care patients, however its use in non-critically ill patients is controversial.1
In recent years, stress ulcer prophylaxis has become increasingly common in internal medicine patients, with scarce or no evidence to support it.1 Acid suppressive medication has been demonstrated to reduce the incidence of clinically significant nosocomial gastrointestinal bleeding in hospitalized patients, both in and outside of the intensive care unit (ICU).2,3 On the other hand, recent studies on the epidemiology of nosocomial gastrointestinal bleeding in non-critically ill patients have found a low overall incidence of 0.3%–0.4% in this setting.2 In addition, safety issues associated with PPIs use have recently attracted attention and several studies reported complications.4 The most recent guidelines available on stress ulcer prophylaxis, published by the American Society of Healthsystem Pharmacists SHP, do not recommend the use of PPIs for medical or surgical patients who are not in an ICU.1,2
We proposed to evaluate the prescription of PPIs as prophylactic measure for preventing nosocomial gastrointestinal bleeding during hospitalization of non-critical care patients, to evaluate the indication for its prescriptions and the associated costs.
Material and Methods
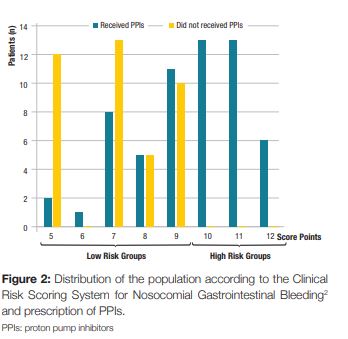
A prospective, observational, cross-sectional study was designed. We included all patients admitted to the Internal Medicine department of our hospital during 31 days (01/03/2016 to 31/03/2016). Before the beginning of the study, a presentation was made to the physicians about PPI use and a proposed clinical score system for predicting the risk of nosocomial gastrointestinal bleeding for hospitalized patients outside of the ICU, published by Herzig SJ et al2 (Table 1). According to this score system, patients were classified into four risk categories for nosocomial gastrointestinal bleeding: low risk (score = 7); low-medium risk (score 8-9); high-medium risk (score 10-11) and high risk (score = 12).2 When the clinical score was =10 points (high-medium or high risk) there was indication for PPI therapy.2 When the clinical score was <10 points had no indication to such therapy.2
The theoretical presentation on PPI use and the score was presented on the month before the study.
During the period of the study, at the date of hospital admission, a questionnaire with this score system was incorporated into the clinical file of each patient. These questionnaires were completed by the attending physician and subsequently collected from the file at discharge. We followed the patients included in order to detect complications such as gastrointestinal bleeding. We asked the hospital pharmacy for data of PPI use and associated costs, during the month of study and during the previous year.
The demographic (age and gender) and clinical data were collected and recorded in the study database. We register the presence or absence of the following clinical data: acute renal failure; liver disease (any disorder of the liver, including acute and chronic hepatitis; acute, subacute, and chronic hepatic failure; chronic liver disease, including hepatic coma, portal hypertension, hepatorenal syndrome and/or other sequelae; hepatic necrosis or infarction; history of liver transplant); sepsis (septicemia due to identified or unidentified organisms, or bacteremia); prophylactic anticoagulation (subcutaneous unfractionated heparin and = 60 mg/day of enoxaparin); and coagulopathy (platelet count < 50.000 cells/µL, or INR > 1.5 or PTT > 2 times control or use of enoxaparin at doses of > 60 mg per day, or fondaparinux).
We used the Statistical Package for the Social Sciences program (SPSS, version 24) as database and statistical analysis. Continuous variables are presented as median, standard deviation (SD), minimum and maximum values, and categorical variables as absolute and relative frequencies.
We excluded all patients in whom the questionnaires were wrongly and/or incompletely filled. This study was approved by the ethics committee of our hospital. Consent requests were not considered necessary.
Results
During the study, 115 patients were admitted to our internal medicine ward. After the exclusion of 16 patients due to incorrect or incomplete filling of the questionnaire, the final evaluation included 99 patients in the analysis (Fig 1). The median age was 76.2 years (SD ± 14.4, range 19 to 101 years), and 54.5% were female (n = 54). Of the risk factors included in the bleeding score, 85.9% of the patients were older than 60 years (n = 85), 45,5% were males (n = 45) and obviously 100% were hospitalized in an internal medicine department. Furthermore, 28.3% of the included patients had acute renal failure (n = 28), 10.1% had liver disease (n = 10) and 20.2% had a diagnosis of sepsis (n = 20). Regarding the prophylactic anticoagulation for deep vein thrombosis it was prescribed in 40.4% patients (n = 40). We found that 30.3% of the patients had coagulopathy (n = 30), based on laboratory values or medication. Therefore, according to the clinical risk scoring system for nosocomial gastrointestinal bleeding2 applied in our study, we found that 67.7% (n = 67) of patients had a score below 10 points (low risk groups) and 32.3% (n = 32) of the patients were in the high-risk groups, with a score equal or above 10 points. (Fig 2). Prophylactic PPIs were prescribed in 59.6% of patients (n = 59), of which 45.8% (n = 27) had no indication to such therapy. On the other hand, 40.4% (n = 40) of the patients on the total population study did not receive PPI prophylactic therapy, of which all had a score below 10 points, so none of them had indication for PPI therapy (Fig 1).
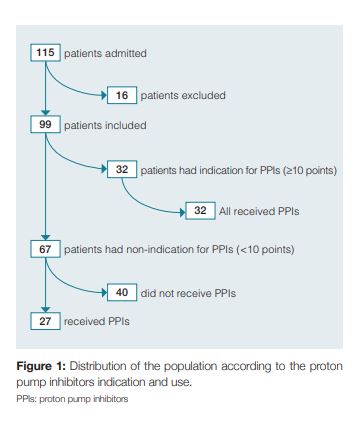
There were no reports of gastrointestinal bleeding in the patients included during the study period. In the evaluation of consumption of PPI, the drugs used were esomeprazol (20 /40 mg, oral or endovenous) or pantoprazol (20/40 mg, oral or endovenous). We verified that during the study 101.9 were spent on PPIs in our internal medicine department (1355.7 in 1 year). The average cost per patient treated with a PPI was 1.7. Considering that the rate of inappropriate use of the drug is 45.8%, we estimated an inappropriate spending of 46.6 in the month of the study in our department.
Discussion
PPIs are commonly used in internal medicine patients which represents a current problem associated with iatrogenic risk and economic impact. Our study demonstrates that PPI use is prevalent among non-ICU medical inpatients at our hospital. We found that 68% of patients had a low/medium-low hemorrhagic risk according to the clinical risk scoring system for nosocomial gastrointestinal bleeding applied in our study,2 and therefore had no indication to receive this therapy. We concluded that the rate of inadequate use of PPIs as a prophylactic measure was 45.8% in our study. Several trials have demonstrated the inappropriate use of acid suppressive therapy in internal medicine patients, based on current recommendations.1 Nardino et al reported the overprescription of acid-suppressive therapy in a large community in an United States hospital where 54% of patients received acid-suppressive therapy, 65% of which were inappropriate.5 Parente et al reported a total of 46.8% of 799 hospitalized patients received acid-suppressive therapy.6 Overall, 68% of prescriptions were inappropriate in hospitalized patients receiving acid- -suppressive therapy and 46% were still receiving the treatment 3 months later.6 In Europe, Gullotta et al described, in a single day study of hospitalized patients at 20 centers in Italy, that 26.8% of hospitalized patients were under PPI treatment, of which 51.4% was inappropriate.7 In Portugal, Fonseca et al published a retrospective analysis that included 511 patients and demonstrated that among hospitalized patients, 89% of patients started acid suppression therapy during the hospitalization, and of these 76% were unnecessary.8 Ribeiro S. et al published a prospective observational cohort study, of 343 patient admissions during an two-month period, in which 186 (54%) patients received PPIs prophylactically, and from this group, 39.8% did not met the criteria for its use.9 Strong evidence supporting PPI efficacy and a favorable safety profile may have contributed to significant overprescription.10 Our study found that almost 60% of the admitted patients had PPIs prescribed, of which 45.8% did not meet criteria. Although the attending physicians had been alerted to the clinical criteria for IBPs use in stress ulcer prophylaxis, there was a high rate of inadequate use of IBPs in our study. There were no reports of gastrointestinal bleeding in the patients included during the study period.
On the other hand, there may be certain subsets of non-critically care patients in whom the risk of nosocomial gastrointestinal bleeding is high enough that prophylactic use of acid-suppressive medication may be warranted.2 We found that 32% of the patients had indication for PPIs and all these patients received PPIs. In 2006 Qadeer et al published a retrospective case-control study designed to identify risk factors that would predict hospital-acquired GI bleeding.11 The major risk factor identified in the study was treatment with any anticoagulant (warfarin, full-dose unfractionated heparin, or full-dose low-molecular weight heparin) or clopidogrel.11 More recently, Herzig et al2 sought the same goal in a large cohort of non-critically ill hospitalized patients, and used this information to develop a clinical risk scoring system.2 The risk score for each patient was derived by summing the risk points for each risk factor present: age over 60 years, male gender, acute renal failure, liver disease, sepsis, prophylactic anticoagulation, coagulopathy and hospitalization in a Medicine department.2 Risk of nosocomial gastrointestinal bleeding increased by more than tenfold from the lowest to highest risk group.2 In our study all patients in this high risk group received PPI therapy.
PPIs have been a widely prescribed drug in internal medicine wards. However, mounting evidence demonstrates that PPIs are associated with a number of adverse effects.4
Available evidence suggests that PPI use is associated with an increased risk of chronic kidney disease,12 acute kidney disease possibly mediated through acute interstitial nephritis,13 hypomagnesemia,14 Clostridium difficile-associated infection,15 and a modest association between PPI use and increased risk of hip and vertebral fractures.16 Reduced gastric acidity and increased bacterial colonization in the stomach related to PPI use may also lead to increased rates of pneumonia.4 The assessment of this complications in our population would be interesting.
The cost of inappropriate use of PPIs was assessed in our study. Considering that the rate of inappropriate use of the drug is 45.8%, we estimated an inappropriate spending of 46.6 during a 31 day period in our Internal Medicine department alone. The evaluation of hospital costs could reveal more significant values. Heidelbaugh JJ and Inadomi JM published a retrospective study about the economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU patients in the United States, over a consecutive 4-month period, including 1769 patient admissions, 22% received stress ulcer prophylaxis which cost $11.024 over the 4 months of the study ($44.096 annually).17
There are several limitations in our study. This is a small study and future studies may be warranted to confirm our results in larger and more representative populations. Additional information including the gastrointestinal background and previous therapeutics with IBPs would have been desirable. The assessment and recording of new risk factors during the hospitalization might have identified changes in the risk score. The evaluation of complications associated with IBPs use was not assessed.
In conclusion, in this study, PPI use was prevalent in non-critically ill patients (59.6%), of which 45.8% were inappropriate. Our results are consistent with published literature. PPIs are commonly inappropriately used in internal medicine patients which represents a current problem associated with iatrogenic risk and economic impact. We recommend adherence to a GI bleeding risk score before the prescription of PPI for prophylactic purposes.
References
1. Grube RRA, May DB. Stress ulcer prophylaxis in hospitalized patients not in intensive care units. Am J Health Syst Pharm. 2007; 64:1396-400. http://dx.doi.org/10.2146/ajhp060393. [ Links ]
2. Herzig SJ, Rothberg MB, Feinbloom DB, Howell MD, Ho KK, Ngo LH, et al. Risk factors for nosocomial gastrointestinal bleeding and use of acid-supressive medication in non-critically ill patients. J Gen Intern Med.2013; 28:683-90. http://dx.doi.org/10.1007/s11606-012-2296-x. [ Links ]
3. Ye ZK, Liu Y, Cui XL, Liu LH. Critical appraisal of the quality of clinical practice guidelines for stress ulcer prophylaxis. PLoS One. 2016; 11: e0155020. http://dx.doi.org/10.1371/journal.pone.0155020. [ Links ]
4. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med.2016;176:172-4. http://dx.doi.org/10.1001/jamainternmed.2015.7927. [ Links ]
5. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000; 95: 3118-22. http://dx.doi.org/10.1111/j.1572-0241.2000.03259.x. [ Links ]
6. Parente F, Cucino C, Gallus S, Bargiggia S, Greco S, Pastore L, et al. Hospital use of acid-suppressive medications and its fall-out on prescribing in general practice: a 1-month survey. Aliment Pharmacol Ther. 2003; 17: 1503-06. [ Links ]
7. Gullotta R, Ferraris L, Cortelezzi C, Minoli G, Prada A, Comin U,et al. Are we correctly using the inhibitors of gastric acid secretion and cytoprotective drugs? Results of a multicentre study. Ital J Gastroenterol Hepatol. 1997; 29: 325-9. [ Links ]
8. Fonseca T, Lopes D, Barreto P, Andrade L, Costa C, Dias.V. Inibição da secreção ácida num Serviço de Medicina Interna. Rev Port Med Int. 2013; 20: 61-7. [ Links ]
9. Ribeiro S, Bathy J, Trabulo D, Cremers MI, Oliveira AP, Pedroso ME. Uso inapropriado de inibidores da bomba de protões num serviço de medicina interna. GE J Port Gastrenterol. 2014;21:9-14. [ Links ]
10. Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Ther Adv Gastroenterol. 2012; 5: 219–32. http://dx.doi.org/10.1177/1756283X12437358.
11. Qadeer MA, Richter JE, Brotman DJ. Hospital-acquired gastrointestinal bleeding outside the critical care unit: risk factors, role of acid suppression, and endoscopy findings. J Hosp Med. 2006; 1:13-20. http://dx.doi.org/10.1002/jhm.10. [ Links ]
12. Lazarus B, Yuan C, Wilson FP, Sang Y, Chang AR, Coresh J,et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016 ; 176: 238-46. http://dx.doi.org/10.1001/jamainternmed.2015.7193. [ Links ]
13. Yang Y, Geoge KC, Shang WF, Zeng R, Ge SW, Xu G. Proton-pump inhibitors use, and risk of acute kidney injury: a meta-analysis of observational studies. Drug Des Devel Ther. 2017;11: 1291-9. http://dx.doi.org/10.2147/DDDT.S130568 [ Links ]
14. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Srivali N, Edmonds PJ, Ungprasert P, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015; 37: 1237-41. http://dx.doi.org/10.3109/0886022X.2015.1057800. [ Links ]
15. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107:1011-9. http://dx.doi.org/10.1038/ajg.2012.108 [ Links ]
16. Ngamruengphong S, Leontiadis GI, Radhi S, Dentino A, Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol 2011; 106:1209–18. http://dx.doi.org/10.1038/ajg.2011.113.
17. Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006;101: 2200-5. http://dx.doi.org/10.1111/j.1572-0241.2006.00839.x. [ Links ]
Correspondência:Ana C. Isabel Chora Sousa aicsousa@gmail.com
Serviço de Medicina Interna, Hospital do Espírito Santo de Évora, Évora, Portugal
Largo Senhor da Pobreza, 7000-811 Évora
Conflitos de Interesse: Os autores declaram a inexistência de conflitos de interesse na realização do presente trabalho.
Fontes de Financiamento: Não existiram fontes externas de financiamento para a realização deste artigo.
Direito à Privacidade e Consentimento Informado: Os autores declaram que nenhum dado que permita a identificação do doente aparece neste artigo.
Proteção de Seres Humanos e Animais: Os autores declaram que não foram realizadas experiências em seres humanos ou animais.
Proveniência e revisão por pares: Não comissionado; revisão externa por pares.
Conflicts of interest: The authors have no conflicts of interest to declare.
Financing Support: This work has not received any contribution, grant or scholarship.
Confidentiality of data: The authors declare that they have followed the protocols of their work center on the publication of data from patients.
Protection of human and animal subjects: The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Provenance and peer review. Not commissioned; externally peer reviewed
Recebido: 28/06/2018
Aceite: 09/10/2018














