Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Medicina Interna
Print version ISSN 0872-671X
Medicina Interna vol.26 no.2 Lisboa June 2019
CASOS CLÍNICOS / CASE REPORTS
Unusual Manifestations of a Rare Clinical Entity: Erdheim-Chester Disease
Manifestação Incomum de uma Entidade Rara: Doença de Erdheim-Chester
Nuno Martins
 https://orcid.org/0000-0003-2510-0043
https://orcid.org/0000-0003-2510-0043
Miguel Achega
 https://orcid.org/0000-0002-5500-0097
https://orcid.org/0000-0002-5500-0097
Alice Rodrigues
 https://orcid.org/0000-0003-3781-5329
https://orcid.org/0000-0003-3781-5329
Fernando Aldomiro
 https://orcid.org/0000-0003-4719-8117
https://orcid.org/0000-0003-4719-8117
Serviço de Medicina II, Hospital Professor Doutor Fernando Fonseca, Amadora, Portugal
Abstract:
A 69-year-old woman presented with sudden left hemiparesis. Computed tomography (CT) scan excluded acute brain injuries. Patient was admitted with acute ischemic stroke of right hemisphere. There was full recovery within 24 hours and etiologic studies were normal. There was a concomitant history of ingestion of 6 litres of water per day over the previous 2 years. Water deprivation test followed by the administration of desmopressin confirmed central diabetes insipidus diagnosis. Brain magnetic resonance imaging (MRI) was normal. Full body CT scan showed arterial wall thickening suggestive of large and medium vessels vasculitis, retroperitoneal fibrosis, perinephric infiltration, pleural and pericardial thickening and diffuse densification of greater omentum. Angio-MRI showed occlusive micro-arterial injuries. Laparoscopic biopsy of greater omentum revealed multinucleated giant Touton CD68+, CD1A- cells. This finding is pathognomonic of Erdheim-Chester Disease, a rare non-Langerhans histiocytosis.
Keywords: Diabetes Insipidus, Neurogenic; Erdheim-Chester Disease; Polydipsia; Retroperitoneal Fibrosis; Stroke
Resumo:
Mulher de 69 anos admitida por hemiparésia esquerda súbita tendo a tomografia computadorizada (TC) encefálica excluído lesões agudas. Internada com o diagnóstico de acidente vascular cerebral isquémico. O quadro neurológico reverteu completamente em menos de 24 horas e o estudo etiológico revelou-se normal. Apurou-se uma história concomitante de ingestão de 6 litros de água por dia nos últimos 2 anos e a prova de restrição hídrica seguida pela administração de desmopressina confirmaram diagnóstico de diabetes insípida central. A ressonância magnética (RM) encefálica foi normal. TC de corpo revelou espessamento da parede arterial sugestivo de vasculite de grandes e médios vasos, fibrose retroperitoneal, infiltração perinefrítica, espessamento pleural e pericárdico e densificação difusa do grande epíploon com o estudo por angio-RM revelando lesões oclusivas micro-arteriais. Biópsia laparoscópica do grande epíploon revelou células gigantes multinucleadas de Touton CD68+, CD1A-, patognomónicas de doença de Erdheim-Chester, uma histiocitose não-Langerhans rara.
Palavras-chave: Acidente Vascular Cerebral; Diabetes Insípida Neurogénica; Doença de Erdheim-Chester; Fibrose Retroperitoneal; Polidipsia
Introduction
Erdheim-Chester disease (ECD) is a rare, non-Langerhans histiocytosis, first described in 1930, with 650-1000 cases reported worldwide,1,2 characterized by xanthomatous infiltration of tissues by CD68-positive, CD1a-negative foamy histiocytes.2 Since 2016 ECD is considered as a histiocyte neoplasm, which means it is a slow-growing blood cancer. Probably, it has its origin in Langerhans cells, bone marrow derived haematopoietic stem and progenitor cells, monocyte and dendritic cell precursors.3,4 The mean age of2 diagnosis is 56.1 years ± 14.7 with male predominance. It is a true multisystem disease as almost all tissues may be infiltrated.1 The multi-organ involvement with important clinical manifestations, major impact in life expectancy and quality makes ECD a diagnostic challenge.
Case Report
A 69-year-old female with no known previous medical disorders presented to the Emergency Department with sudden left hemiparesis.
Brain computer tomography (CT) had no acute pathological findings and the patient was admitted to Internal Medicine ward with the clinical diagnosis of right hemisphere ischemic stroke. There was a total recovery within 24 hours.
Holter and Doppler ultrasound of neck vessels were normal; echocardiogram revealed pericardial thickening.
A thorough clinical history revealed large water consumption, about 6 litres per day, over the past two years.
Blood chemistry revealed: hypernatremia (147 mmol/L), erythrocyte sedimentation rate 114 mm/1st hour, serum osmolality 293 mOsm/Kg and urinary osmolality 144 mOsm/kg.
For confirmation of diabetes insipidus it was done a water deprivation test. After 7 hours of water deprivation, plasma osmolality roseto 303 mOsm/kg (criteria for stopping the test) and urinary osmolality to 190 mOsm/kg (Table 1).
Desmopressin was administered to test central diabetes insipidus. Four hours after administration of subcutaneous desmopressin (2 mcg), plasma osmolality dropped to 294 mOsm/Kg, urine osmolality increased to 366 mOsm/kg and urinary volume was only 70 cm3 (Table 2).
Brain MRI revealed normal hypophysis dimensions, but semioval centre had bilateral hyperintensities (probable micro-arterial occlusive aetiology) and both middle cerebral arteries had stenosis of M1 segment.
Because of arterial findings in brain MRI, it was requested a cervico-thoraco-abdomino-pelvic CT scan, which revealed parietal thickening of all major arteries, including carotid arteries (Fig 1a), thoracic aorta (Fig 1b), abdominal aorta (Fig 1c) and superior mesenteric artery. It also revealed pulmonary parenchyma band densifications and bilateral thickening of pleura. Pyelocaliceal system parietal thickening, perinephrium adipose tissue densification and greater omentum retroperitoneal fibrosis and densification were additional findings.
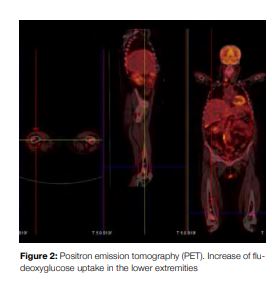
Fluorodeoxyglucose (FDG) positron emission tomography confirmed CT scan findings as well as distal uptake of bone marrow in both femurs (Fig 2).
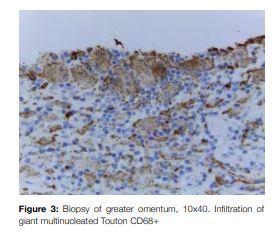
Laparoscopic surgery was carried out revealing greater omentum multiple white implants which were biopsied finding giant multinucleated Touton CD68+, CD1a- cells (Fig 3).
Being these findings pathognomonic of Erdheim-Chester disease, the patient started treatment with pegylated interferon alpha.
Discussion
In summary, this patient was a 69-year-old woman with a multi-organ disease. The brain had vascular involvement as seen in the brain MRI and central diabetes insipidus. She had arterial involvement with parietal thickening of small, middle and large vessels. The lungs had band densification of parenchyma and pleural thickening and the heart had also pericardial thickening. The kidneys had pyelocaliceal and perinephric adipose tissue densification. There was retroperitoneal fibrosis and densification of the greater omentum and the bones were also affected as seen by the alterations in bone marrow of both femurs.
The differential diagnosis included, at first, an autoimmune aetiology, specifically a vasculitis, which is classically divided into small, middle or large vessel. This patient presented parietal thickening of all arteries, but antineutrophil cytoplasmic antibodies were negative, as were anti-nuclear antibody, Waller Rose reaction, anti-Scl-70, anti-CCPe, anti-ß2glicoprotein, anti-cardiolipin, lupus anticoagulant, anti-SSA and SSB, anti-dsDNA, anti-nucleosomes, anti-histones and anti-RNP/SM.
An infectious aetiology was also excluded as blood tests for human immunodeficiency virus (HIV), hepatitis B, hepatitis C, Epstein-Barr virus, parvovirus B19, herpes simplex I and II, VDRL and IGRA were all negative.
The next step was infiltrative diseases as sarcoidosis or histiocytosis. For sarcoidosis the angiotensin converting enzyme was negative and the biopsies of the greater omentum implants revealed infiltration of giant multinucleated Touton CD68+, CD1a- cells, which are pathognomic findings of Erdheim-Chester disease and excluded sarcoidosis.
ECD is a rare multi-systemic disease of unknown aetiology. Its most common clinical finding is bone pain, affecting 50% of patients, since only 4% ECD patients present without symmetric osteosclerosis of the femurs. However this manifestation is unspecific at the age this diagnosis is usually made (40-70 years old).
The frequency of central nervous system (CNS) involvement varies from 25% - 50%. Infiltration can occur throughout the neuroaxis and can be intra or extra-axial. Symptoms reflect compression of local structures or deterioration of cognitive functions and gait in the presence of diffuse disease. Diabetes insipidus is present in 25% of patients.2,5 CNS involvement is a strong prognostic factor and an independent predictor of death in cases of ECD.1,6
As there were no acute ischemic findings in the brain MRI and the left hemiparesis had full recovery, we assumed the concomitant diagnosis of transient ischemic attack. The arterial findings pointed to our final diagnosis.
Central diabetes insipidus was diagnosed by water deprivation test and desmopressin test. There were no hypothalamic or pituitary changes on the brain MRI but with desmopressin test positive, it does not exclude the diagnosis.
Perinephric fat infiltration, known as ´hairy kidney´ is present in 68% of cases.
In 30% of patients, images suggestive of retroperitoneal fibrosis are present.
Cardiovascular involvement is usually asymptomatic and detected incidentally by CT scan. The most common finding is infiltration of thoracic and abdominal aorta and respective branches. Pericardial disease may be present in 40% - 45% of patients and can present as pericarditis, effusion or tamponade.
Lung involvement is present in 50% of ECD patients, presenting as interlobular septal thickening, ground-glass opacities or centrilobular opacities.
These findings are usually asymptomatic, but some patients may present with cough or dyspnoea. Our patient presented with all these clinical findings and, according to the proposed ECD classification,2 the diagnosis is a symptomatic multisystem ECD.
There have been few studies and no randomized clinical trials about treatment of ECD. Several treatment options were tried, including anti-cytokine directed therapy (anakinra, infliximab, tocilizumab), corticosteroids and radiotherapy.4,5,7-11
However, since the therapeutic modality with the largest supporting efficacy evidence is pegylated IFN-alpha, this was our choice.2,5
The recent finding that 57% - 75% of ECD patients carry BRAFV600E mutations made a way to other therapeutic options with serine/threonine kinase inhibitors, such as vemurafenib, which has become the reference second-line treatment. However, our patient did not present this mutation.1,12
Prognosis was unfavourable before treatment with interferon, with only 43% of patients being alive 32 months after diagnosis.13 This approach, however, increased this proportion to 82.8% at 5 years.1
In this age of mutation targeted pharmacology, the finding that other recurrent mutations of the MAPK and PIK3 pathways (NRAS, PIK3CA) are present in ECD patients can lead to new drugs that might, considerably, improve prognosis.1
References
1. Haroche J, Cohen-Aubart F, Charlotte F, Maksud P, Grenier P, Cluzel P, et al. The histiocytosis Erdheim-Chester disease is an inflammatory myeloid neoplasm. Expert Rev Clin Immunol. 2015;11:1033-42. doi: 10.1586/1744666X.2015.1060857. [ Links ]
2. Diamond EL, Dagna L, Hyman DM, Cavalli G, Janku F, Estrada-Veras J, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124:483-92. doi: 10.1182/ blood-2014-03-561381. [ Links ]
3. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms Blood. 2016;127:2375-90. doi: 10.1182/blood-2016-01-643569. [ Links ]
4. Haroche J, Cohen-Aubart F, Rollins BJ, Donadieu J, Charlotte F, Idbaih A, et al. Histiocytoses: emerging neoplasia behind inflammation. Lancet Oncol. 2017;18:e113-e125. doi: 10.1016/S1470-2045(17)30031-1. [ Links ]
5. Haroche J, Arnaud L, Amoura Z. Erdheim–Chester disease. Curr Opin Rheumatol. 2012;24:53–9.
6. Arnaud L, Hervier B, Néel A, Hamidou MA, Kahn JE, Wechsler B, et al. CNS involvement and treatment with interferon- are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011;117:2778-82. doi: 10.1182/blood-2010-06-294108. [ Links ]
7. Cives M, Simone V, Rizzo FM, Dicuonzo F, Cristallo Lacalamita M, Ingravallo G, et al. Erdheim–Chester disease: A systematic review. Crit Rev Oncol Hematol. 2015;95:1-11. doi: 10.1016/j.critrevonc.2015.02.004.
8. Tran TA, Pariente D, Lecron JC, Delwail A, Taoufik Y, Meinzer U. Treatment of pediatric Erdheim-Chester disease with interleukin-1- targeting drugs. Arthritis Rheum. 2011;63:4031-32. doi: 10.1002/art.30638. [ Links ]
9. Killu AM, Liang JJ, Jaffe AS. Erdheim-Chester disease with cardiac involvement successfully treated with anakinra. Int J Cardiol. 2013;167:e115-7. doi: 10.1016/j.ijcard.2013.04.057. [ Links ]
10. Dagna L, Corti A, Langheim S, Guglielmi B, De Cobelli F, Doglioni C, et al. Tumor necrosis factor a as a master regulator of inflammation in Erdheim-Chester disease: rationale for the treatment of patients with infliximab. J Clin Oncol. 2012;30:e286-90. [ Links ]
11. Jendro MC, Zeidler H, Rosenthal H, Haller H, Schwarz A. Improvement of Erdheim-Chester disease in two patients by sequential treatment with vinblastine and mycophenolate mofetil. Clin Rheumatol. 2004;23:52-6. [ Links ]
12. Haroche J, Cohen-Aubart F, Emile JF, Arnaud L, Maksud P, Charlotte F, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495-500. doi: 10.1182/blood-2012-07-446286. [ Links ]
13. Veyssier-Belot C, Cacoub P, Caparros-Lefebvre D, Wechsler J, Brun B, Remy M, et al. Erdheim-Chester disease. Clinical and radiologic characteristics of 59 cases. Medicine. 1996;75:157-69. [ Links ]
Correspondência:Nuno Martins - nunomartins2@gmail.com
Serviço de Medicina II, Hospital Professor Doutor Fernando Fonseca, Amadora, Portugal
44IC19, 2720-276 Amadora
Conflitos de Interesse: Os autores declaram a inexistência de conflitos de interesse na realização do presente trabalho.
Conflicts of interest: The authors have no conflicts of interest to declare.
Fontes de Financiamento: Não existiram fontes externas de financiamento para a realização deste artigo.
Financing Support: This work has not received any contribution, grant or scholarship.
Direito à Privacidade e Consentimento Informado: Os autores declaram que nenhum dado que permita a identificação do doente aparece neste artigo.
Confidentiality of data: The authors declare that they have followed the protocols of their work center on the publication of data from patients.
Proteção de Seres Humanos e Animais: Os autores declaram que não foram realizadas experiências em seres humanos ou animais.
Protection of human and animal subjects: The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Proveniência e revisão por pares: Não comissionado; revisão externa por pares.
Provenance and peer review. Not Commissioned; externally peer reviewed.
Recebido: 12/10/2018
Aceite: 23/01/2019














