Introduction
Behçet´s disease (BD) was first described in 1937, by Hulusi Behçet, as a triad of recurrent oral and genital aphthosis and uveitis.1 BD is a systemic vasculitis of unknown cause, classified among the variable vessel vasculitis,2 that may affect almost every organ.
Although BD has a worldwide distribution, its prevalence is higher along the ancient “´silk route” extending from the Mediterranean region to the Middle East and Far East countries.3-6Based on a national sample in 1997 that included 241 patients, in Portugal, a prevalence of 2.4 cases / 100 000 inhabitants was estimated.7
BD´s organ involvement and clinical course exhibit different phenotypes across different regions, although the factors that lead to these differences are not clear.3-5,8Although the BD´s pathogenesis is unclear, some studies have shown the possible contribution of the inflammatory response initiated by infectious agents or autoantigens in patients with predisposing genetic factors and perpetuated by innate and acquired immunity dysregulation.9-12Besides, a network of immune mediators, resulting in numerous cytokines and chemokines production, such as tumor necrosis factor-alpha (TNF-α), plays a substantial role in the inflammatory cascade, activates macrophages, and is responsible for cell-mediated immunity.12,13
The first reference to BD treatment with a biological drug, specifically an anti-TNFα agent (infliximab), dates back to 2001 in patients with severe panuveitis.14 Several studies have proven biologicals effectiveness in different manifestations since then. Anti-TNFα are currently recommended in severe BD forms,15,16namely mucocutaneous involvement refractory to non-biological therapy, ocular involvement affecting the posterior chamber or recurrent episodes of uveitis, refractory venous thrombosis, arterial or central nervous system (CNS) involvement, severe or refractory gastrointestinal involvement and chronic or refractory joint involvement.
This study aims to describe the outcomes after biological therapy in BD patients in a single-centre cohort, as well as the complications that arose from it.
Methods
SETTING, PATIENTS AND STUDY DESIGN
We carried out a prospective, longitudinal cohort study including all patients with diagnostic criteria for BD according to the International Study Group in 1990,17 followed in the outpatient clinic of Clinical Immunology Unit, Centro Hospitalar e Universitário do Porto, between January-1981 and September-2020.
Patients were followed from the moment the diagnosis was established, and the manifestations were recorded as they arise. Hospital Ethics Committee approved the study design, and informed consent was waived due to its observational nature (study number 2020-162 (127-DEFI-129-CE)).
DATA AND DEFINITIONS
We collected data regarding demographic features and symptoms of BD: oral aphthosis (OA), genital aphthosis (GA); skin manifestations - erythema nodosum (EN), pseu-dofolliculitis; joint manifestations - arthralgias, monoarthritis, polyarthritis, spondyloarthritis; ocular, neurological, vascular, gastrointestinal and cardiac involvement. Ocular manifestations include uveitis, retinal vasculitis, amaurosis, papillitis, conjunctivitis, and scleritis. Neurological manifestations include hemispheric, brainstem, and spinal cord syndromes, dural sinus thrombosis, and CNS arterial occlusion. Vascular manifestations include deep vein thrombosis, thrombophlebitis, arterial aneurysms, and arterial occlusion of other than CNS vessels. Esophageal, gastroduodenal, intestinal, and perianal ulcers, digestive bleeding from ulcers, bowel perforation, and chronic inflammatory diarrhea were reported as gastrointestinal manifestations. Behçet´s Disease Current Activity Form (BDCAF) was used to score disease activity in patients receiving biologics, presented as transformed index score. We also collected data related to therapies carried out, duration of those therapies, clinical response, and complications. The presence of HLA-B*51 allele was also collected.
STATISTICAL ANALYSIS
Categorical variables are presented as an absolute value and relative frequency, and the numerical variables as mean + standard deviation (SD). For comparison between groups, the Pearson Chi-square test was used for categorical variables or the Fisher´s exact test; the Student t-test was used for numerical variables. BDCAF score change over time was presented in a boxplot graph and compared with a paired sample t-test. All statistical tests were 2-sided. Significance level as set to 0.05. Statistical analyses were performed using SPSS software (SPSS for Windows, version 26, Chicago, IL, USA).
Results
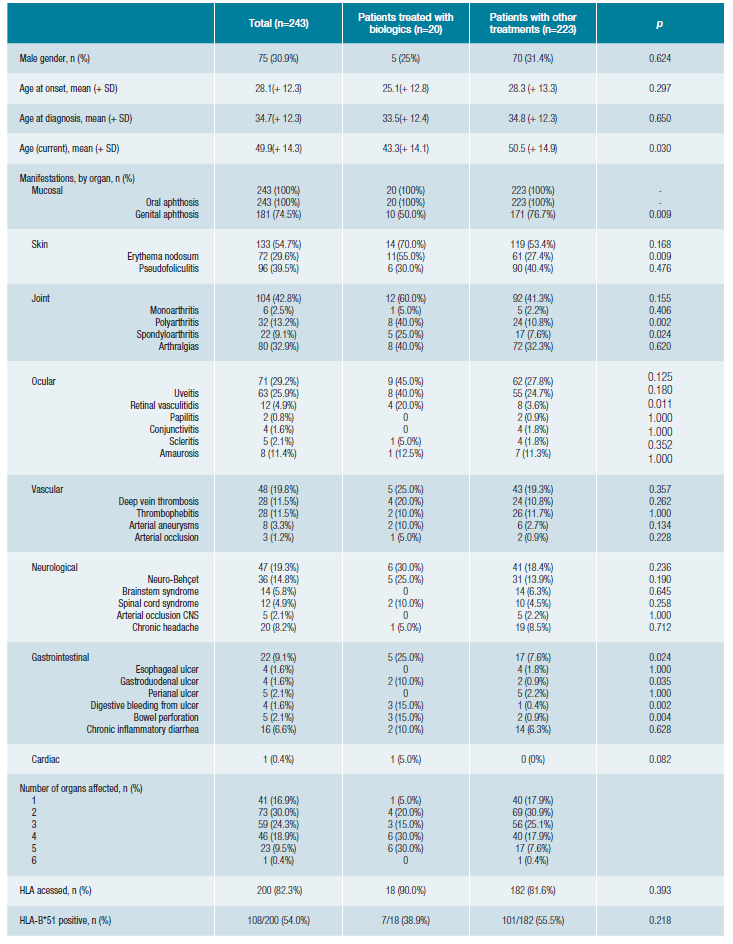
Our cohort includes 243 patients, of which 75 (31%) pa-tients are male. The current age was 49.9 (+14.3) years old. The first signs and symptoms of the disease started at 28 (+12.3) years old, and patients were diagnosed as having BD at 34.7 (+12.3) years old. The HLA-B*51 allele was only ac-cessed in 82% (n = 200) patients and positive in 54% (n = 108).
Regarding organ manifestations (Table 1), all patients had mucous manifestations, 55% skin, 43% joint, 29% ocu-lar, 20% vascular, 19% neurological, 9% gastrointestinal and 0.4% cardiac BD involvement. Most patients have three or more organs affected by BD.
During follow-up, 20 patients (8%) were treated with biological drugs. Comparing the patients who received biologi-cal treatment with the patients who received other treatments (Table 1), we found that they were younger (p = 0.030), had less frequently GA (p = 0.009), more frequently EN (p = 0.009), polyarthritis (p = 0.002), spondyloarthritis (p = 0.024), retinal vasculitis (p = 0.011) and gastrointestinal manifestations (p = 0.024), namely gastroduodenal ulcer (p = 0.035), digestive bleeding (p = 0.002), and bowel perforation (p = 0.004). Overall, patients who received biological treatment have more organs affected (p = 0.022).
Tabela 1: Demographic and clinical characteristics of patients with Behçet's disease (BD) and comparison between patients treated with biologics and patients treated with other drugs.
Of the 20 patients requiring biological therapy to control BD, most had only severe manifestations afflicting one organ (Table 2). One patient (patient 1) needed biological therapy twice during the follow-up period for flares in two organs, neurological and gastrointestinal, in different periods.
In this cohort, all the organ manifestations of BD are represented. The organ involvement that induced the biological therapy starting were heterogeneous (Table 2) (20 patients, n=21 organ involvement): articular (29%, n = 6), gastrointestinal (19%, n = 4), ocular (14%, n = 3), neurological (14%, n = 3), vascular (10%, n = 2), mucocutaneous (9%, n = 2) and cardiovascular (5%, n = 1). On the other hand, the proportion of patients in need of biological therapies was higher in patients exhibiting cardiac (1/1, 100%) and gastrointestinal (4/22, 18%) manifestations.
Table 2: Clinical characteristics of Behçet's disease (BD) patients treated with biological therapies.
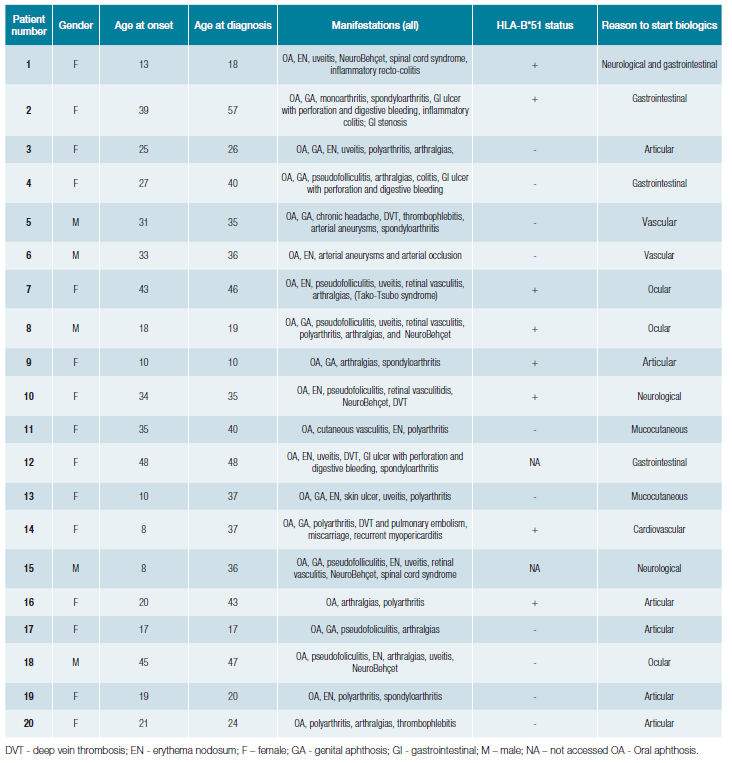
In our centre, biologicals have been used in BD since 2008. The first case was a 32-year-old man with recurrent uveitis, unresponsive to cyclosporine (patient 8) who underwent complete remission and maintained therapy (infliximab) for ten years, until loss of efficacy due to the appearance of anti-infliximab antibodies.
During the follow-up period, these 20 patients (Table 2) had 23 flares motivating therapy with 30 biological courses (Table 3). The most frequent biological agent used was infliximab. All patients started anti-TNFα agents after classical im-munosuppressors failure to control BD, except for patient 18, who started infliximab in another country, and so, we do not have information regarding previous treatments attempted.
Table 3: Biological therapies used during follow-up in patients with Behçet's disease and clinical outcomes. Each line represents a biological therapy course.
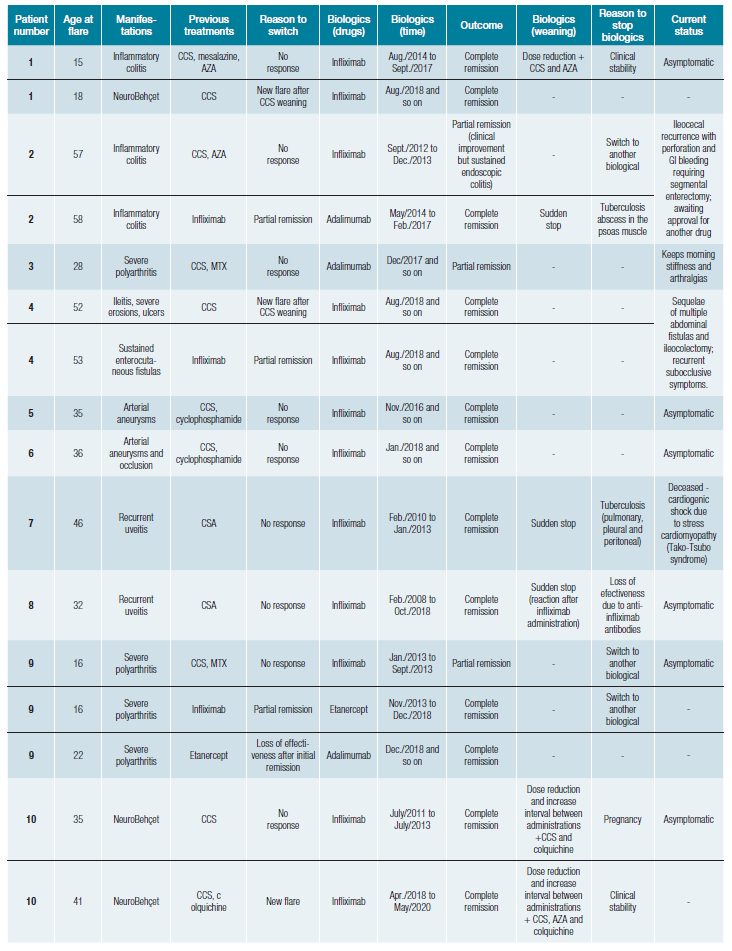
Table 3 (cont.): Biological therapies used during follow-up in patients with Behçet's disease and clinical outcomes. Each line represents a biological therapy course.

After starting the biological drug, the patients underwent complete remission in 22 cases (73%) and six into a partial remission (20%) (Table 3), with a mean BDCAF reduction of 4 points at 12 months after (Fig. 1). Eight patients underwent switch to another biological: three that presented a partial remission, two patients motivated by symptom worsening, a primary failure, and additionally, three other patients, with secondary failure: initial complete remission with disease recurrence a few months after, assumed as drug effectiveness loss (both 33 months after, in two patients with infliximab; and 61 months in one patient with etanercept).
Three patients had anti-TNFα related complications with tuberculosis (two with infliximab and one with adalimumab) (Table 3). Of these patients who developed tuberculosis, all had previous screening tests. Patient 2 have had Pott´s disease in her youth, had undergone treatment for latent tuberculosis before starting infliximab, and was being screened with an annual tuberculosis-interferon gamma release assay test (IGRA). During follow-up, the patient presented a positive IGRA and then was diagnosed with a tuberculosis abscess, despite asymptomatic. Another patient, patient 7, had sustained negative IGRA tests before and during infliximab treatment and developed disseminated tuberculosis. Albeit had unsuspected screening test, tuberculin skin test (TST), and standard chest radiography prior to infliximab, patient 16 developed pleural tuberculosis in less than six months after starting the anti-TNFα agent.
Currently, 12 patients are still in biological therapy (Table 3). Anti-TNFα therapy was stopped in seven patients (patients 1, 2, 8, 12, 13, 17, and on patient 10 twice). Two patients had BD recurrence grounding a new biological therapy course: patient 1, with a neurological flare 11 months after stopping infli-ximab for gastrointestinal BD; and patient 10, who had a new neurological flare 58 months after infliximab suspension. Therapy was stopped in one patient due to tuberculosis develo-pment, despite active BD gastrointestinal manifestations. Five patients remained in remission so far, with a follow-up of 33 months (DP+20) after suspension: 23 months for ocular BD (patient 8); 4 months for a neurological flare (patient 10); 24 months in a gastrointestinal BD case (patient 12); 45 months for mucocutaneous involvement (patient 13); and 63 months in articular BD (patient 17). In most cases, biological therapy weaning followed a period of dose reduction and increased intervals between administrations, together with other classic immunosuppressants (Table 3). On the other hand, in patients who experienced an adverse drug reaction or infectious com-plications such as tuberculosis, biological therapy has been suddenly stopped.
One patient died during the follow-up period (patient 7). Two patients were awaiting approval for a new biological drug.
Discussion
BD´s therapeutic approach should be primarily tailored to disease severity and based on the nature of organ involve-ment in order to identify the most appropriate treatment choice. More severe manifestations can be faced with biological drugs in order to counteract irreversible organ damage and life-threatening complications.18 In our centre, the patients started biological due to a panoply of manifestations that include all BD,s organ damage.
Over the past few years, there has been an increase in the use of biological therapies in patients with refractory manifestations of BD. The cumulative experience is leading to a transition from the old classic therapies considered until now as the gold standard to newer drugs.18 Among these, anti-TNF-α agents have been increasingly employed showing to be effective in all severe BD manifestations resistant to conventional therapy.16,18,19In our centre, all patients who underwent biological treatment had received an anti-TNF-α agent, and biological therapy followed classical immunosuppressors failure. Most of our patients went into BD remission (93%) after starting biological drugs, with significant symptoms decrease, bearing witness to these therapies effectiveness.
Some authors describe cases of BD recurrence with life-threatening manifestations after biologicals are withdrawn.20,21In our centre, we have tried to suspend the biological therapy in seven patients, in which, five of them successfully so far. Two patients had BD recurrence after suspension, both with a neuro-Behçet syndrome, requiring a new biological course. Therefore, frequent monitoring of these patients is necessary after anti-TNF-α cessation in order to an earlier detection of relapses. Also, it will be important to also extend the time of surveillance in clinical trials with anti-TNFα agents as most studies only address treatment outcomes within a few weeks after biologics introduction. On the other hand, bearing in mind that these therapies are not innocuous, very little is known regarding optimal biological therapy duration after achieving a long-standing complete remission, when and how to stop therapy in order to avoid relapses.
Despite a regular latent tuberculosis screening, before and during the biological therapies well established in our centre, three patients developed tuberculosis during treatment. The risk is higher in anti-TNF-α agents,22 given the role of this cytokine in the pathogens of mycobacterial infections. TNF-α is required for the granuloma formation and inhibition of the reactivation of dormant bacilli.23 Along with these therapies, it is essential to emphasize the need for a regular screening test without despising a high clinical suspicion. On the other hand, both screening tests evaluate cell-mediated immunity and can give false-negative results in immunosuppressed patients. Nowadays is recommended to use both screening tests in these patients (TST and IGRA) together with clinical assessment.24 Of notice, in one case, the diagnosis was made in an asymptomatic patient who presented a positive screening test, and in another patient who presented an extensive disease despite negative scree-ning tests.
Our study´s main strengths lie in the fact that we have considered all BD´s organ manifestations refractory to con-ventional therapies, not limited to a specific organ. Also relevant is the high number of BD patients followed at our centre, and the experience with biological therapies for over ten years. The main weaknesses of this study are due to its observational nature with its inherent limitations and with the fact that these patients represent only 8% of our sample.
Conclusion
BD is a systemic inflammatory disorder in which several cytokines appear to play a substantial role. Biological targeted therapies are highly effective in controlling refractory BD´s manifestations, although they are not innocuous. Currently, little is known about the optimal duration of these therapies after the achievement of long-standing complete remission, regarding when and how to stop these drugs. This is important not only to avoid relapses that can some-times be life-threatening but also to reduce the deleterious therapy side-effects.
PREVIOUS PRESENTATIONS
The authors declare that this work was partially presented at the Global Summit of Internal Medicine/XLIII Congresso Nacional de Medicina Interna 2020, Mexico, December 2020.















