Introduction
Neutrophil-to-lymphocyte ratio (NLR) at hospital admis-sion, defined as the quotient between both absolute values in a complete blood count, has been suggested to be a promising marker in cardiovascular ischaemic events, such as coronary artery occlusion.1,2A higher NLR was independently associated with arterial stiffness and coronary calcium score in a large Korean study,3 proposing that NLR might be a useful additional measure of assessing cardiovascular (CV) risk. In fact, a high NLR despite a normal white blood cell (WBC) count is proposed to be predictive of atherosclerosis,4 and a more powerful predictor of CV disease than any leukocyte subtype,5 presumably due to the role of inflammation in the atherogenic process.6 It has also been associated with a poor short-term clinical outcome in patients with acute ischaemic stroke7,8and hemorrhagic stroke,9,10on par with other inflammatory biomarkers.11-13
Post-stroke pneumonia (PSP) is defined as a lower respiratory tract infection complicating the first week after stroke onset,14 the period where pneumonia most frequently occurs in stroke patients.15 This fact probably reflects the period of highest risk in terms of dysphagia, immobility, impaired consciousness, and immunosuppression.16,17It has an estimated incidence of 7% to 38%15,18-27and has been consistently associated with a high attributable risk of early mortality, increased length of stay and medical cost.18,22,28-30As such, prompt identification of patients at high risk for PSP is clinically relevant by promoting an increased monitoring and potential tailored prophylactic or therapeutic measures.31-33
The A2DS2 score - a ten-point score (age ≥75 years=1, atrial fibrillation=1, dysphagia=2, male sex=1, stroke severity, National Institutes of Health Stroke Scale NIHSS 0-4=0, 5-15=3, ≥16=5) developed from the Berlin Stroke Registry, is currently the most used tool. It was validated in the independent Northwest Germany Stroke Registry 34, and with external validation 35. A prospective multicentre comparison between A2DS2, ISAN and AIS-APS scores (also commonly used to predict the risk of PSP), suggest that A2DS2 might be the best score in the identification of patients at high risk of PSP, though none with a good positive predictive value. In fact, a A2DS2 score of ≥4 yields a sensitivity of 91% and specificity of 57% for the occurrence of PSP, while a A2DS2 score ≥5 has a sensitivity of 83% and specificity of 72%.34
The authors aim to assess the relationship between NLR and stroke severity, subtype, how it relates to mortality, and post-stroke pneumonia incidence.
Material and Methods
A prospective observational study over a 42-month period in a Stroke Unit of a tertiary University Hospital was conducted. All patients presenting with acute ischemic stroke during this period were included. Patients that underwent thrombolysis or mechanical thrombectomy were not included. Population and event characteristics were collected, namely: time of onset, type of event, Oxfordshire Community Stroke Project - OCSP, National Institute of Health Stroke Scale - NIHSS, mortality, mRankin at discharge and length of stay in days), risk factors such as chronic obstructive pulmonary disease, heart failure or active smoking, presence of respiratory tract infections, A2DS2 and NLR at admission to the emergency department. NLR cut-off was selected based on previous published methodology.36
STATISTICAL ANALYSIS
Statistical analysis was performed using SPSS® statistical software version 24. Relevant variables were stratified in cate-gories (A2DS2 < 6, >= 6; NIHSS at admission <6, 6-13, >13; NLR <3, ≥3). When comparing two categorical variables the Pearson’s Chi-square test was used. A binomial logistic re-gression multivariate analysis was also performed. Applicabi-lity conditions were verified. The significance level was set at p < 0.05.
Results
A total of 606 patients were admitted to the Stroke Unit during the study’s period. Of these, 521 patients with acute ischemic stroke (AIS) were identified (Table 1). The remaining 85 patients were excluded due to being hospitalized with ei-ther a haemorrhagic event or a stroke mimic. Of the included 521 patients classified as AIS, 66 had a transient ischaemic attack (TIA).
Table 1: Diagnosis at admission
| Type of Event | Frequency |
|---|---|
| Ischaemic stroke | 455 |
| Transient ischaemic attack | 66 |
| Haemorrhagic stroke | 21 |
| Stroke mimic | 64 |
| Total | 606 |
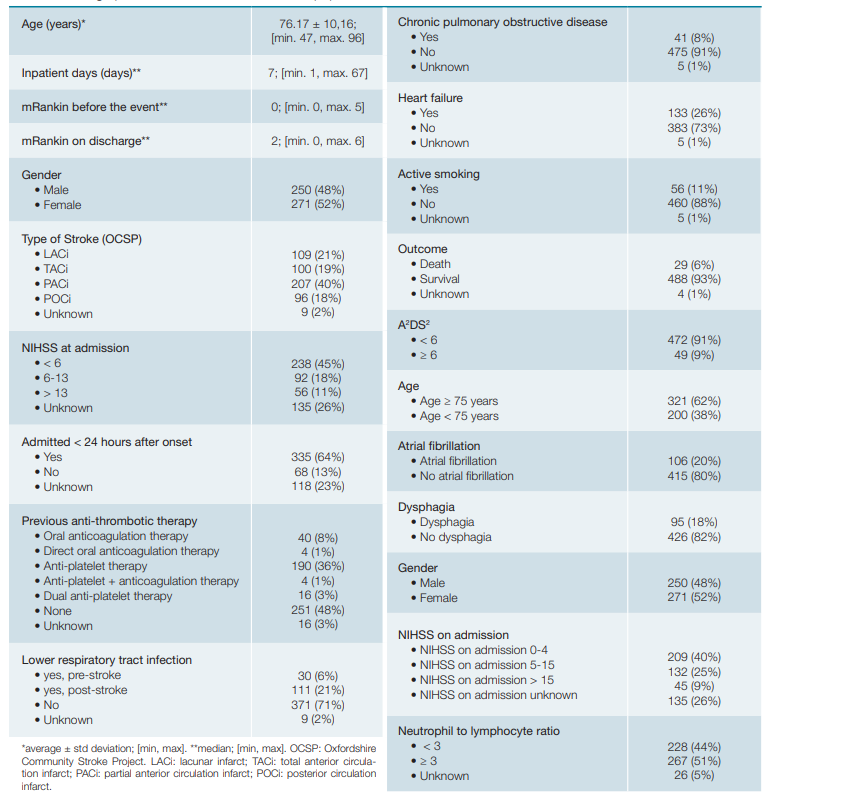
Concerning the included patients, the mean age was 76.17 ± 10.16 years (min. 47; max. 96 years old). Population demographics are summarized on Table 2. Missing data was characterized as unknown.
Association was found between NLR and type and seve-rity of stroke according to OSCP classification - lower NLR in lacunar stroke and higher in total anterior circulation infarct (Table 3, p < 0.001).
Table 3: Comparison between low and high NLR and stroke according to OSCP, death and mRankin at discharge.
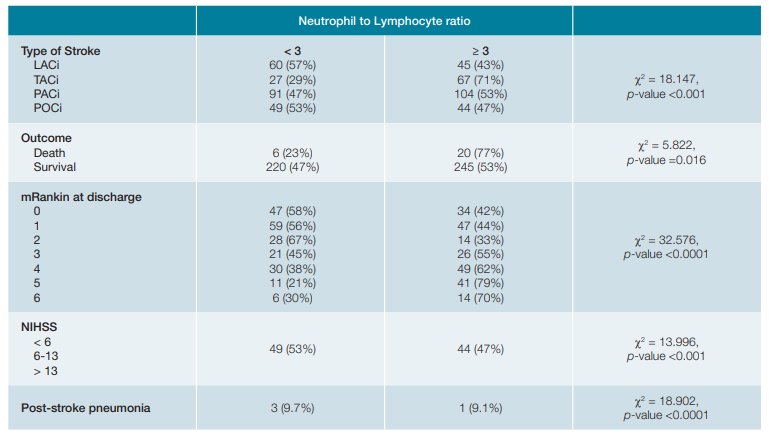
A NLR ≥3 was related to more severe neurological deficit as ascertained by the NIHSS scale ≥6 (p < 0.001) and was associated with a higher mortality - from 26 patients that died, 77% had a NLR ≥ 3, vs 52% of those in the survival group (Table 3, p = 0.016). NLR ≥ 3 was also associated with higher disability as ascertained by mRankin score at discharge (Table 3, p < 0.0001). This association persisted after stratified analy-sis excluding infection (PSP).
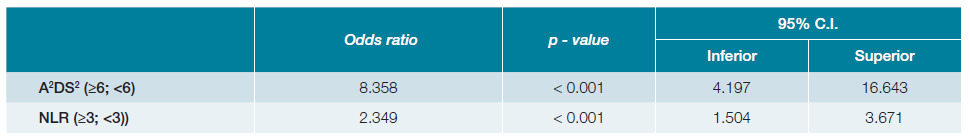
The relationship between NLR at admission and the deve-lopment of pneumonia is shown in Fig. 1. Patients with NLR ≥ 3 had higher odds of developing pneumonia (OR 2.531, 95%CI 1.654, 3.873). Since a statistically significant relationship between NLR and post-stroke pneumonia was found, a logistic regression was performed to assess the predictive capacity of NLR (≥3) and A2DS2 (≥6) on the likelihood of developing post-stroke pneumonia. The model including both variables explained 17.1% (Nagelkerke R2) of the variance in pneumonia diag-noses, correctly classifying 77.0% of patients and showing a 96.3% specificity and a 25.6% sensibility (p < 0.005), with a better fitness than a model using A2DS2 alone (13.2%, Nagelkerke R2). The specificity and sensibility found in our sample with A2DS2 alone was similar. The positive predicti-ve value was 72.3% and the negative predictive value was 77.4%.
A2DS2 ≥ 6 was 8.36 times more likely to exhibit post-stroke pneumonia than A2DS2 <6 (p < 0.001), while NLR ≥ 3 had 2.349 higher odds (Table 4).
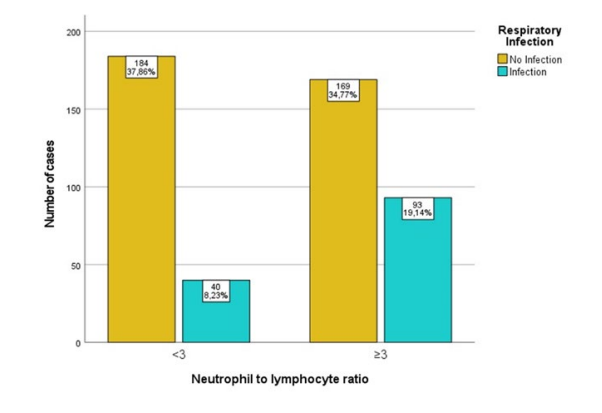
Figure 1: Relationship between NLR at admission and post-stroke pneumonia. Missing.35. p-value < 0.0001. X2 = 18.902. OR 2.531, 95%CI (1.654, 3.873), unadjusted.
Discussion
There is little doubt that post-stroke inflammation is an important factor in brain injury.17,37-39In fact, leucocytosis has been associated to a poorer clinical outcome in patients with AIS.40-43Nevertheless, this inflammatory response is complex and involves several protagonists and immune pathways.17,38NLR has been suggested as an interesting marker in patients with cardiovascular disease.44,45It has been associated with a poor short-term clinical outcome in patients with acute ischaemic stroke7,8and is also a marker of poor prognosis 3 months after AIS.46 In fact, the immune system might arise as a potential therapeutic target in patients with ischaemic brain lesion.47-49
In our study, there was an association between higher NLR, higher NIHSS and poorer outcome, with increased risk of mortality and a higher mRankin score at discharge. These results persisted after stratified analysis excluding infection.
Post-stroke pneumonia is a frequent but potentially pre-ventable stroke complication, often associated with a significant increase in morbidity and mortality. It has been associated with a 4 times higher fatality rate in the first 30 days, 3 times higher length of stay and a significant increase in medical expenses.16,30Our incidence of 21% falls within what has been reported in the literature.15,18-27 NLR is an established marker for inflammation and infection, having been studied as a predictor in pneumonia and bacterial infections50-53However, its role in PSP is not as well supported. In a cohort of 1317 patients in a 2-center retrospective study, Nam K-W, et al report an association between NLR and an increased risk and severity of PSP.54 We also report an association between higher NLR and an increased risk of developing PSP.
Several clinical risk factors have previously been established for PSP, such as age, admission NIHSS score and stroke severity, presence of nasogastric tube, mechanical ventilation, and atrial fibrillation.55 However, the concomitant use of bio-markers in PSP assessment is, to the best of our knowledge, scarce.27,55,56The PANTHERIS score included the total leukocyte count, but the lack of NIHSS evaluation seems to be a limitation.27 In our study, patients with NLR ≥ 3 were 2.35 times more likely to exhibit post-stroke pneumonia than NLR < 3 (p <0.001) and NLR did appear to increase the fitness of the predictability of A2DS2 in our logistical regression model, even though we did not find an increase in sensitivity or specificity in our sample.
The percentage of “unknown data”, namely in NIHSS, is attributed to logistical constraints in our practice setting: a tertiary centre involving two hospitals. Study inclusion was done at stroke unit admission in a different hospital from the emergency department (ED). When the details from the stroke physician’s evaluation were not charted at the ED, data was classified as unknown/missing.
Post-stroke inflammation is characterized by a rapid ac-tivation of resident cells (mainly microglial cells), followed by the infiltration of circulating inflammatory cells, including gra-nulocytes (neutrophils), T cells, monocyte/macrophages, and other cells in the ischemic brain region.57-59Microglial cell proliferation and proinflammatory mediator production, including IL-1β and TNF-a, has been documented within minutes of ischemia60 and appear to play a role in exacerbating tissue damage but may also protect the brain against ischemic and excitotoxic injury.60,61In contrast, blood-derived leukocytes are recruited to the brain tissue with a delay of hours to a few days. Therefore, the timing of blood collection could affect NLR. NLR evolution during hospitalization was not evaluated due to concern of higher risk of bias due to infection, catheterization, and drugs. The NLR response in a severe cardiovascular event appears to be a surrogate of an immune-mediated response, not necessarily caused by infection but perhaps related to it (higher immune-dysregulation in more severe events).62
This, in our view, supports the current hypothesis of immune-mediated cell injury in stroke. Further studies are needed in determining the immunological mechanism of lesion and the possibility of therapeutic intervention.
Conclusion
NLR ≥3 is associated with a more severe stroke subtype, neurologic deficit, increased morbidity and mortality and higher rates of post-stroke pneumonia, though the relationships described do not seem to be attributable do the infection. NLR appears to be a surrogate of immunological dysregulation. Advances in the knowledge of the immunobiological effects of ischemia in brain may lead to future therapeutic developments. As an inexpensive, fast and widely available tool, NLR may have a role in identifying a subset of patients that may benefit in future immunomodulatory therapy trial.
Declaração de Contribuição / Contributorship Statement:
D. Pedro, M. Narciso - Conceção e design, Análise e recolha de dados, Análise estatística e Interpretação de dados, Escrita do artigo, Pesquisa Bibliográfica, Revisão crítica do artigo, Aprovação final.
M. Alves, T. Fonseca - Conceção do artigo, Pesquisa bibliográfica, Revisão crítica do artigo, Aprovação final.