Introduction
Community-acquired pneumonia (CAP) is a frequent cause of hospitalization, particularly in the elderly population, which has been growing exponentially.1,2Several studies have shown that CAP is associated with significant morbidity and mortality, both in the short- and long-term.3,4Acute kidney injury (AKI) can occur as a complication of CAP in at least 18% to 34% of the patients, even in CAP considered non-severe, having shown association with a worse prognosis, including greater severity of pneumonia, length of hospital stay and mortality.1,5,6Although not yet fully understood, the pathophysiological mechanisms underlying the development of AKI in sepsis have been extensively investigated,7,8contrary to the relationship between AKI and less severe infections without evidence of sepsis.5 In general terms, it has been demonstrated that the development of AKI is a multifactorial phenomenon, in which inflammatory and immunological processes may play a key role.1,5-7On the other hand, some factors, including advanced age, chronic kidney disease (CKD), diabetes, use of diuretics and statins, have shown association with an increased risk of developing AKI in the context of CAP, although data available is scarce and contradictory.9-11Early identification of patients with a higher risk of AKI in CAP allows a more adequate and timelier preventive and therapeutic approach, with potential improvement of the prognosis.5,9
The main objective of this study was to identify independent associated factors for the development of AKI in CAP, among baseline patient characteristics and gasometric parameters. Secondary objectives were to estimate the incidence and assess the impact of AKI in CAP in terms of length of hospital stay, in-hospital and post-discharge mortality, and survival.
Material and Methods
We conducted a retrospective cohort study in patients hospitalized in the Internal Medicine department of Centro Hospitalar e Universitário de Coimbra, from November 2018 to October 2019. Patient status, alive or deceased, after discharge was evaluated until 31st of January 2022. This study was performed in accordance with the Declaration of Helsinki of the World Medical Association and was approved by the Ethics Committee of the Centro Hospitalar e Universitário de Coimbra.
Among all patients admitted in the department in the above-mentioned time period, we included patients with a definitive diagnosis of pneumonia on admission, according to the presence of new consolidation or infiltrate on chest radiograph accompanied by at least two signs or symptoms of pulmonary infection, including fever, cough, sputum, shortness of breath, chest pain, pulmonary auscultation changes and low oxygen levels.
Exclusion criteria included hospital-acquired pneumonia, aspiration pneumonitis/pneumonia, pulmonary embolism, end-stage kidney disease, patients under dialysis, immunosuppressive therapy or not treated with antibiotics. To reduce bias, we also excluded patients with other possi-ble causes of AKI, mainly other sources of infection, acute or decompensated chronic heart failure (HF), decompensated cirrhosis, non-septic shock, as well as intrinsic or postrenal AKI.
The following variables were obtained: age, sex, dependency for activities of daily living (ADLs), comorbidities, Charlson Comorbidity Index (CCI), usual medication before admission, symptoms, vital signs, including blood pressure (BP), heart rate (HR), temperature, respiratory rate (RR), and oxygen saturation, pulmonary auscultation findings, basal and on admission creatinine, urea nitrogen and gasometric values on admission, length of hospital stay, status at discharge, and survival. AKI was defined and classified using the Kidney Disease/Improving Global Outcomes (KDIGO) criteria,12 considering the creatinine value on hospital admission. CAP was classified on admission using validated scores CURB-65 and Pneumonia Severity Index (PSI) and was defined as non-severe CAP if PSI classes I to III, or severe if classes IV and V.5
Outcomes for prognosis evaluation were: in-hospital mortality, 30 day, 6-month and 1-year mortality, and length of hospital stay. All clinical and laboratory characteristics were collected from the hospital electronic clinical records and were anonymously stored.
Statistical analysis was performed using the software IBM SPSS version 24. Descriptive statistics of demographic and clinical variables are presented as absolute and relative frequencies for categorical data, mean and standard deviation (SD) for normally distributed quantitative variables, or median and interquartile range (IQR) for non-parametric methodology. Baseline demographic patient characteristics as well as PSI class, CURB-65 and length of hospital stay, with and without AKI, were compared using the Student’s t-test or Mann-Whitney U-test, depending on the normality of the distribution. The chi-square or Fisher’s exact tests were used for categorical variables.
Variables with potential association with AKI were analyzed using a univariate logistic regression to calculate their unadjusted odds ratio (OR). To create a probabilistic model of AKI in CAP, we ran a multivariable logistic regression using the forward: Wald method. A Hosmer-Lemeshow test was used to evaluate the goodness-of-fit and a receiver operating characteristic (ROC) curve was used to test the discriminative performance of the model. Univariate logistic regression was also used to assess the impact of AKI on mortality rates of CAP. Survival curves were built using the Kaplan-Meier method and comparison of survival in non-AKI and AKI patients was achieved with the logrank test, and the impact of AKI on survival was evaluated using a Cox regression. A two-tailed p value of less than 0.05 was considered statistically significant and a 95% confidence interval (CI) was used.
Results
The original sample consisted of 1066 patients with inclusion criteria, of whom 488 were excluded, according to the previously defined exclusion criteria. A total of 578 patients were ultimately included.
Patients’ general characteristics on admission
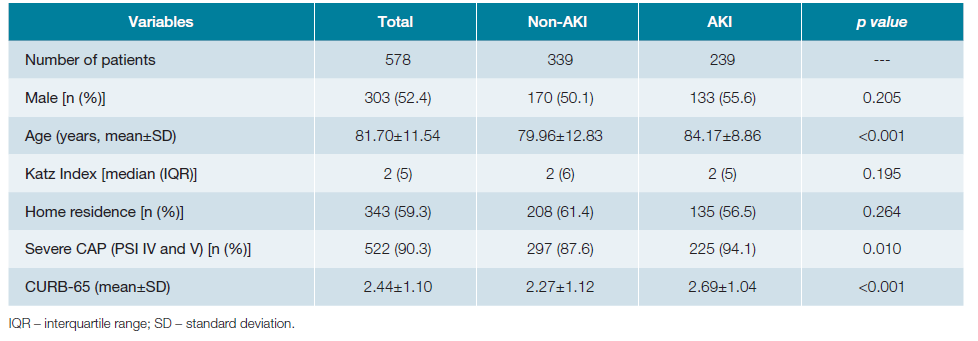
Analyzing the total number of patients, 303 (52.4%) were male, the mean age was 81.70 ± 11.54 years and 200 (34.6%) were fully dependent for ADLs, with a median Katz Index of 2 (IQR 5). Mean creatinine value on admission was 1.26 ± 0.78 mg/dL, ranging between 0.33 and 6.35 mg/dL, with AKI identified in 41.3% (n = 239) of the patients, of which 195 (81.6%) were KDIGO 1, 37 (15.5%) KDIGO 2, and 7 (2.9%) KDIGO 3. Mean basal creatinine levels before admission were 0.95 ± 0.44, ranging between 0.21 and 2.98 mg/dL. Mean urea nitrogen on admission was 31.75 ± 22.04 mg/dL. CAP was classified according to the PSI as non-severe CAP in 56 (9.7%) patients and severe in the remaining 522 (90.3%). The incidence of AKI according to PSI group was 25.0% (n = 14) in non-severe CAP and 43.1% (n = 225) in severe CAP. The general characteristics of the patients distributed according to the absence (non-AKI) or presence of AKI are shown in Table 1. Using comparative tests between the non-AKI and AKI groups, statistically significant differences were observed for age (79.96 ± 12.83 vs 84.17 ± 8.86, p <0.001), severe CAP (87.6% vs 94.1%, p = 0.01) and CURB-65 (2.27 ± 1.12 vs 2.69 ± 1.04, p <0.001).
Factors associated with AKI
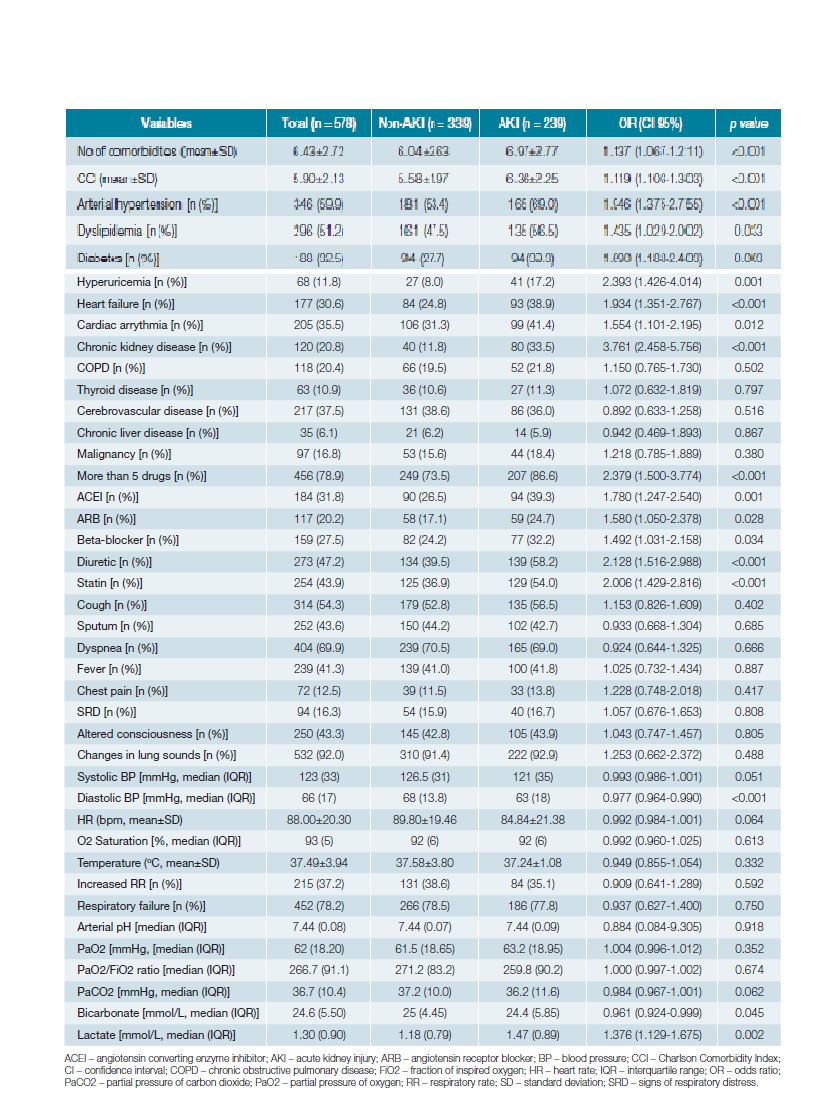
The univariate logistic regression analysis of the variables with potential association with AKI is shown in Table 2. The variables with statistically significant unadjusted ORs were: num-ber of comorbidities (OR 1.137, 95% CI 1.067-1.211), CCI (OR 1.119, 95% CI 1.103-1.303), arterial hypertension (OR 1.946, 95% CI 1.375-2.755), dyslipidemia (OR 1.435, 95% CI 1.029-2.002), diabetes (OR 1.690, 95% CI 1.188-2.403), hyperuricemia (OR 2.393, 95% CI 1.426-4.014), HF (OR 1.934, 95% CI 1.351-2.767), cardiac arrhythmia (OR 1.554, 95% CI 1.101-2.195), CKD (OR 3.761, 95% CI 2.458-5.756), taking more than 5 drugs (OR 2.379, 95% CI 1.500-3.774), use of angiotensin converting enzyme inhibitor (ACEI) (OR 1.780, 95% CI 1.247-2.540), angiotensin receptor blocker (ARB) (OR 1.580, 95% CI 1.050-2.378), beta-blocker (OR 1.492, 95% CI 1.031-2.158), diuretic (OR 2.128, 95% CI 1.516-2.988), statin (OR 2.006, 95% CI 1.429-2.816), diastolic BP (OR 0.977, 95% CI 0.964-0.990), serum bicarbonate (OR 0.961, 95% CI 0.924-0.999) and serum lactate (OR 1.376, 95% CI 1.129-1.675).
Multivariable probabilistic model of aki in cap
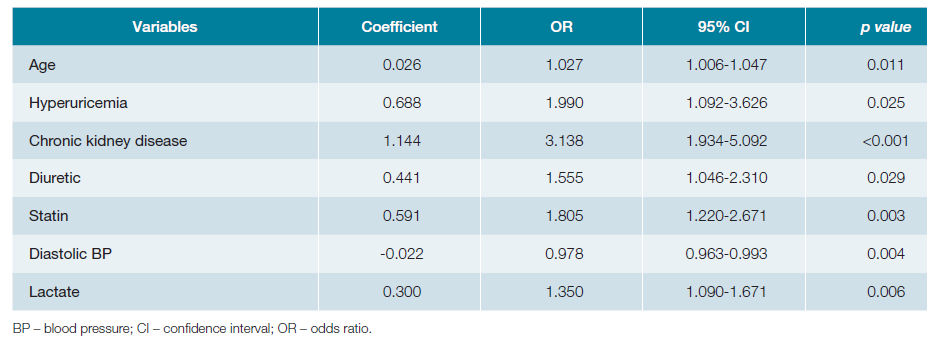
Including the statistically significant variables of the univariate analysis, and adding sex and age, a multivariable logistic regression analysis was performed using the forward: Wald method, allowing the identification of factors associated with AKI with statistically significant adjusted ORs (Table 3): age, hyperuricemia, CKD, diuretic, statin, diastolic BP and serum lactate. Using the respective regression coefficients, we built a probabilistic model of AKI in CAP (Fig. 1), with a sensitivity of 52.7% and a specificity of 81.1%, correctly classifying 68.9% of the cases. The Hosmer-Lemeshow test did not reject the null hypothesis that the predicted values are close to the actual observed values, with a p value of 0.894. The ROC curve obtained an area under the curve of 0.728 (95% CI 0.684-0.771).
Outcomes and survival
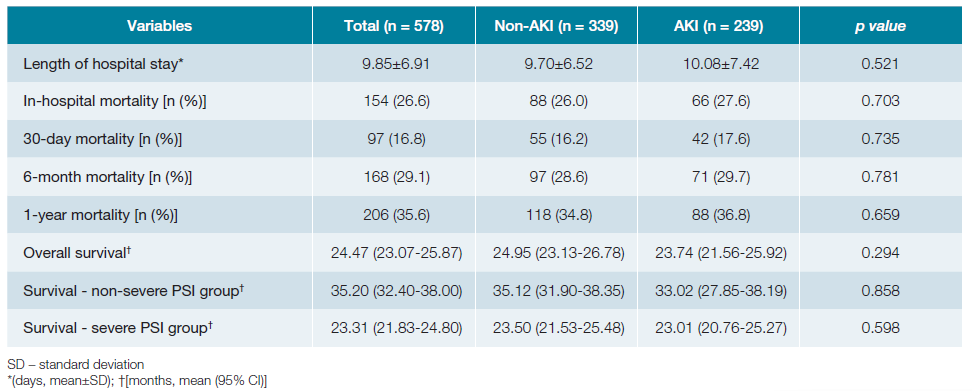
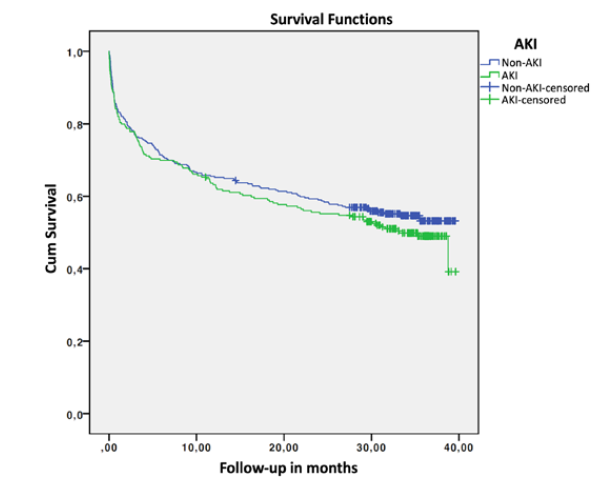
Global in-hospital mortality rate was 26.6% (n = 154), with a mean length of hospital stay of 9.85 ± 6.91 days. The median follow-up time after admission was 29.55 (IQR 31.42) months. Mean survival was 24.47 months (95% CI 23.07-25.87), with a 6-month mortality of 29.1%. Comparison of outcomes and survival in the non-AKI and AKI groups is shown in Table 4. Although the AKI group presented higher mortality, there were no statistically significant differences. Univariate logistic regression to assess impact of AKI on mortality did not obtain significant OR: in-hospital mortality OR 1.088 (95% CI 0.749-1.581, p = 0.657); 30-day mortality OR 1.106 (95% CI 0.712-1.720, p = 0.653); 6-month mortality OR 1.054 (95% CI 0.733-1.517, p = 0.776); 1-year mortality OR 1.091 (95% CI 0.773-1.541, p = 0.619). Fig. 2 shows the survival curves in months for the non-AKI and AKI groups.
Survival was marginally lower in the AKI group, with a non-significant p value in the log-rank test (p = 0.294) and in the Cox regression (p = 0.329, hazard ratio 1.127, 95% CI 0.887-1.431). Adjusting according to the PSI group, the log-rank test continues to obtain p values greater than 0.05 (p = 0.858 and p = 0.598, for non-severe and severe CAP, respectively). In the group of patients with AKI, there were no differences in survival by KDIGO class (p = 0.370).
Discussion
AKI is a relatively frequent complication of CAP, regardless of its severity or the presence of sepsis, having an important impact on short- and long-term prognosis.1,5,6However, the underlying mechanisms are not fully understood, nor are the factors or individuals potentially at risk.5,9-11To the best of our knowledge, this is the first study to assess the incidence, impact and factors associated with AKI in Portuguese patients hospitalized for CAP. In this study, 41.3% of the patients admitted for CAP had AKI on admission, a higher incidence than previously reported.1,5,9,10As expected, since these patients required hospitalization, the majority (90.3%) had a PSI class IV or V, with an incidence of AKI of 43.1% in this group. In the non-severe CAP group (PSI I to III), AKI was identified in 25.0% of the patients, a lower percentage than described in other studies.5
From another point of view, there was a higher percentage of patients with PSI IV or V, as well as a higher mean value of CURB-65 in the group with AKI (p = 0.01 and p <0.001, respectively), which may be due, on the one hand, to the fact that the severity scores include urea nitrogen, but, on the other hand, to the fact that the mechanisms underlying severe pneumonia may contribute to the development of AKI and vice versa, in a process of synergistic interaction between the lung and the kidney.1,6,10,13Although there is still scarcity of data available, some clinical and demographic characteristics have been identified as possible factors associated with AKI, including male gender and advanced age.9,10,14In this study, although the male gender represented a higher percentage in the AKI group versus in the non-AKI, this difference was not statistically significant. On the contrary, the mean age was statistically significantly higher in the AKI group (79.96 ± 12.83 vs 84.17 ± 8.86, p <0.001).
Regarding comorbidities and drugs, previous studies have not obtained entirely concordant results, but diabetes, hypertension, HF and the use of ACEIs or ARBs were the factors most consistently identified.1,5,9,10,14In our univariate logistic analysis, hypertension, dyslipidemia, diabetes, hyperuricemia, HF, cardiac arrhythmia, CKD, use of ACEIs, ARBs, diuretics, beta-blockers and statins were associated with AKI with statistically significant unadjusted ORs. Furthermore, the number of comorbidities, the CCI and taking more than 5 drugs showed the same significant association with AKI, contributing to the evidence that this is a multifactorial process that may depend not only on a specific comorbidity, but on the constellation and interaction of the patient’s previous pathologies.
In contrast, symptomatology on admission did not show any association, not even altered state of consciousness, which could potentially be associated with dehydration, a well-known mechanism of development of AKI. Among the vital signs, only diastolic BP on admission was inversely associated with the development of AKI, with systolic BP showing the same tendency, which can reflect the role of hemodynamic changes in this process. On the other hand, one study identified acute respiratory failure as an independent risk factor for AKI in CAP.10 Based on this assumption, we extended the analysis to other gasometric parameters in arterial blood, considering they are valuable indicators of the lung-kidney interaction in acid-base balance, and are easily accessible on patient admission, often even before any laboratory work result. In our sample, neither the presence of respiratory failure nor the partial pressure of oxygen/fraction of inspired oxygen ratio showed association with AKI, but the serum levels of bicarbonate and lactate proved to be potential factors associated with AKI, the first intrinsically linked to renal function, and the latter a possible hemodynamic marker.
The multivariable logistic regression of the factors identified in the univariate analysis selected age, hyperuricemia, CKD, diuretics, statins, diastolic BP (inverse correlation) and serum lactate as independent factors associated with AKI. Of note, and as far as the authors are aware, hyperuricemia, diastolic BP and serum lactate had not been assessed in previous studies in the specific context of CAP, revealing that there is still a wide range of factors and mechanisms that need to be investigated. Unsurprisingly, CKD is the variable most strongly associated with AKI, having an adjusted OR of 3.138 (95% CI 1.934-5.092). Hyperuricemia also showed substantial association since its presence increased the odds of AKI by a factor of almost 2 (OR 1.990 with a 95% CI of 1.092-3.626). On the other hand, age and diastolic BP showed a poor association, presenting an OR of nearly 1. Based on the results of the multivariable analysis, we built a probabilistic model to calculate the probability of a patient presenting AKI in the context of CAP. Despite having a relatively low sensitivity, the specificity of the model is greater than 80%, and the area under the ROC curve and the Hosmer-Lemeshow test confirmed its adequcy. In addition, this model incorporates a balanced range of variables, by including a demographic characteristic, previous pathologies, drugs, as well as a vital sign and a gasometric parameter. Therefore, this model can be the starting point for the development of internally and externally validated predictive indexes applicable in clinical practice, allowing a fast and timely screening of patients at risk of AKI, who may need closer monitoring of the renal function, as well as the implementation of preventive strategies for the development of AKI.
Finally, unlike what is reported in the literature that the presence of AKI correlates with poor prognosis,1,5,6,9,10in this study, despite presenting a higher mortality, with OR and Hazard Ratio values slightly greater than 1, the development of AKI did not have a statistically significant impact on the length of hospital stay, prognosis and survival, which may be due not only to the fact that most AKI patients were KDIGO 1, but also to a prompt and effective approach by the medical teams.
The main limitation of this study is its retrospective cross-sectional nature, allowing only the identification of associations, but not causal relationships or true risk factors. Additionally, in order to reduce potential confounders associated with hospitalization, only creatinine on admission was evaluated, thus, the development of AKI during the hospital stay was not assessed. On the other hand, we included a relatively high number of patients, enabling a multivariable logistic analysis with statistical value.
Conclusion
This is the first study performed in a sample of Portuguese patients that confirms that AKI is common in patients with CAP, occurring in a higher percentage than previously reported, as well as in PSI classes I to III, a population that is often not included.1,5
Furthermore, it reinforces the importance of comorbidities and previous medications as factors associated with the development of AKI. On the other hand, symptomatology did not correlate with this process, even altered state of consciousness that could be associated with dehydration, proving that there are other underlying pathophysiological mechanisms. In turn, apart from respiratory failure, the analysis of gasometric parameters has not been previously performed in other studies, and we identified serum lactate as an independent associated factor. The multivariable logistic regression model represents a breakthrough in the development of true predictive indexes of AKI in CAP with applicability in clinical practice and impact on the therapeutic approach and prognosis.