Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Jornal Português de Gastrenterologia
versão impressa ISSN 0872-8178
J Port Gastrenterol. vol.19 no.6 Lisboa nov. 2012
https://doi.org/10.1016/j.jpg.2012.07.010
Hepatorenal syndrome, septic shock and renal failure as mortality predictors in patients with spontaneous bacterial peritonites
Síndrome hepatorrenal, choque sético e insuficiência renal como preditores de mortalidade em doentes com peritonite bacteriana espontânea
Eduardo Rodrigues-Pintoa,∗, Margarida Freitas-Silvab
a Gastroenterology Department, Hospital São João, Faculdade de Medicina do Porto, Porto, Portugal
b Internal Medicine Department, Hospital São João, Faculdade de Medicina do Porto, Porto, Portugal
*Corresponding author.
Abstract
Background and aims: Spontaneous bacterial peritonitis (SBP) is a common complication of cirrhosis. Identification of poor prognosis predictors is essential in disease approach.
Methods: Medical records from patients admitted at our institution between January 2008 and December 2010 with spontaneous bacterial peritonitis were retrospectively reviewed. Criteria assessed were age, sex, presenting symptoms, risk factors, ascitic fluid characteristics, evolution during hospitalization, prophylaxis at discharge and re-admission.
Results: 42 (34 male, 8 female) patients were included in the study. Mean age was 57.46± 13.4 years. Abdominal pain was the most common presenting symptom (59.5%); 69% of patients had Child-Pugh C. 7.1% have had previous episodes of spontaneous bacterial peritonitis, but only 2.4% were on antibiotic prophylaxis. 71.4% of first paracentesis were culture-negative. In the remaining, Escherichia coli (16.7%) was the agent most frequently isolated. 32.25% patients who started treatment with Ceftriaxone, were switched to another antibiotic. Average length of hospitalization was 16.10±12 days. Mortality rate was 28.6%. Of the variables analyzed with the methodology of Cox, hepatorenal syndrome (HR = 29.92, p < 0.001) and septic shock (HR = 9.5, p = 0.001) were significantly associated with higher mortality risk, with renal failure being suggestively associated (HR = 3.25, p = 0.063). Of the 71.4% patients discharged, 46.67% were on prophylaxis with 21.42% of them being re-admitted with the same diagnosis, while 31.25% discharged without prophylaxis were re-admitted (p = 0.36).
Conclusion: The mortality is elevated, with hepatorenal syndrome and septic shock being potential predictors of mortality. Ceftriaxone fails in a high percentage of SBP episodes and may not be the most appropriate first-line treatment.
KEYWORDS Peritonitis; Hepatorenal syndrome; Septic shock; Renal insufficiency
Resumo
Introdução & objetivos: A peritonite bacteriana espontanea (PBE) e uma complicação comum em doentes cirroticos. A identificação de fatores preditivos de mau prognostico e essencial na abordagem desta patologia.
Métodos: Foram analisados retrospetivamente os processos clinicos dos doentes internados na nossa instituição entre janeiro de 2008 e dezembro de 2009 com o diagnostico de PBE. Os criterios avaliados foram idade, sexo, sintomas de apresentação, fatores de risco, características do liquido ascitico, evolução no internamento, medicação profilatica aquando da alta e reinternamento.
Resultados: Foram incluidos 42 doentes no estudo (34 do sexo masculino e 8 do sexo feminino). A idade media no internamento foi 57,46±13,4 anos. A dor abdominal foi o sintoma de apresentação mais comum (59,5%); 69% dos doentes tinham Child-Pugh C. 7,1% ja tinham tido episodios previos de peritonite bacteriana espontanea, mas apenas 2,4% fazia profilaxia antibiotica a admissao. Nao se isolou qualquer agente em 71,4% dos doentes; nos restantes, a E. coli foi o agente mais frequente (16,7%). 32,25% dos doentes que iniciaram antibioterapia empirica com ceftriaxone tiveram que alterar para outro antibiotico com maior espetro de acção. A duração media do internamento foi de 16,10±12 dias. A taxa de mortalidade foi de 28,6%. Das variaveis analisadas com a metodologia de Cox, estao significativamente associadas a risco de mortalidade mais elevado o sindrome hepatorrenal (HR = 29,92; p<0,001), o choque setico (HR = 9,5; p = 0,001) e sugestivamente a insuficiencia renal (HR = 3,25; p = 0,063). Dos 71,4% doentes que tiveram alta, 46,67% foram medicados profilaticamente, com 21,42% a serem reinternados com o mesmo diagnostico, sendo que apenas 31,25% dos doentes que tiveram alta sem profilaxia foram reinternados (p = 0,36).
Conclusão: A mortalidade e elevada, sendo a presença de sindrome hepatorrenal e choque setico potenciais preditores de risco de morte. O ceftriaxone pode nao ser o antibiotico empirico de primeira linha mais adequado, tendo em conta a falencia terapeutica numa percentagem elevada de doentes.
PALAVRAS-CHAVE Peritonite; Sindrome hepatorrenal; Choque setico; Insuficiencia renal
Introduction
Spontaneous bacterial peritonitis (SBP) is a common and severe complication in patients with advanced cirrhosis. It is defined as an ascitic fluid infection without an evidente intra-abdominal cause. When first described, its mortality rate exceeded 90% but with early diagnosis and treatment it is now reduced to about 20%.1,2
The diagnosis of SBP is established with a diagnostic paracentesis.3 All patients with cirrhosis and ascites are at risk of SBP; its prevalence is higher in hospitalized patients (10% versus 1.3-3.5%).4 Half of the patients are diagnosed with SBP at hospital admission and the rest consist in nosocomial infections.
Ascites culture is negative in as many as 60% of patients with clinical manifestations suggestive of SBP and increased ascites neutrophil count.3,5 For SBP diagnosis, the number of polymorphonuclear leucocytes in the ascitic fluid must exceed 250 cells/mm3 and bacteriological cultures must be positive.3,6 Patients with an ascitic fluid neutrophil count >250 cells/mm3 and negative culture have culture-negative SBP. Their clinical presentation is similar to that of patients with culture-positive SBP and should be given the same treatment.3,6 Some patients have bacterascites in which cultures are positive but ascitic fluid neutrophil count is <250/mm3.3,6 Bacterascites may result from secondary bacterial colonization of ascites from an extraperitoneal infection or from spontaneous colonization of ascites, and it can be a transient and spontaneously reversible colonization of ascites, or may represent the first step in the development of SBP.
The most common pathogens involved are Gram-negative bacteria (60%), usually Escherichia coli or Klebsiella pneumonia.3,6,7 In about 25% of the cases, Gram-positive bacteria are involved, mainly Streptococcus species and Enterococci.7,8 This is manly due to the prophylaxis with quinolones, used to reduce the incidence of SBP episodes.9 Although the bowel flora is predominantly anaerobic, these microorganisms rarely cause SBP.7 The epidemiology of bacterial infections differs between community-acquired (in which Gram negative infections predominate) and nosocomial infections (in which Gram-positive infections predominate).6
The clinical presentation in SBP is non-specific. Patients, particularly outpatients, may be asymptomatic. Other signs and symptoms associated include fever, abdominal pain, chills, nausea or vomiting, ileus, diarrhea, mental status changes and renal impairment.
Antibiotics should be started at diagnosis and adjusted, if necessary, according with the ascitic fluid cultural results.
Considering Gram-negative bacteria are the most frequente pathogens involved, the first line antibiotic treatment should be third-generation cephalosporins.10-12 Alternative options include amoxycillin/clavulanic acid, quinolones and piperacilin/tazobactam. SBP resolves with antibiotic therapy in approximately 90% of patients. A second paracentesis, 48 h after the beginning of antibiotic therapy, should be made to assess a decline in the neutrophil count, when no clinical improvement occurs or when the initial ascitic fluid analysis revealed atypical findings.11 Failure of antibiotic therapy is usually due to resistant bacteria or secondary bacterial peritonitis.
Certain subgroups of patients with cirrhosis and ascites have a higher risk of developing SBP and should be on a prophylaxis antibiotic regimen. The use of prophylactic antibiotics is approved in patients with acute gastrointestinal hemorrhage, patients with low total protein concentration in ascitic fluid (and no prior history of SBP) and patients with a previous history of SBP.7 Newer quinolones are the prophylactic antibiotics of choice because they not only eliminate aerobic Gram-negative bacteria from the intestinal flora but also appear to have an immunoregulatory part by stimulating the bactericidal capacity of polymorphonuclear cells and decreasing bacterial adhesion to mucosal surfaces.11
Approximately half of all deaths in patients with SBP occur after the resolution of the infection and are usually the result of gastrointestinal hemorrhage, liver or renal failure. The presence of renal failure is the strongest independente prognostic indicator, but the presence of peripheral leukocytosis, advanced age, higher Child-Pugh score and ileus have also shown to predict inpatient mortality.13-19 Patients with nosocomial versus community-acquired SBP appear to have a higher mortality. The existence of a positive ascitic fluid culture or bacteremia does not seem to influence prognosis.13
The aim of this study was to characterize a consecutive series of patients with SBP diagnosis, regarding risk factors, complications during hospitalization and their influence in prognostic.
Methods
Medical records from patients admitted between January 2008 and December 2009 with the diagnosis of SBP (either at admission or during hospitalization) were reviewed. The criteria assessed were:
- Patients age and gender;
- Symptoms at presentation (as fever, abdominal pain, changes in gut motility and mental status);
- Risk factors for SBP (severity of liver disease according to Child-Pughs classification, prior episodes of SBP, presence of esophageal varices, use of proton pump inhibitors, if on an antibiotic prophylactic regimen, total serum bilirubin concentration >2.5 mg/dL, plasma creatinine ≥1.2 mg/dL, plasma sodium ≤130 mEq/L);
- Characteristics of the ascitic fluid (cytochemical SBP criteria, ascitic fluid culture, ascitic fluid total protein concentration <1.5 g/dL);
- Clinical evolution (if complicated by septic shock, hepatorenal syndrome, renal failure or other infections and length of hospitalization);
- Antibiotic therapy (if discharged on a prophylactic regimen);
- Re-admissions with the same diagnosis (the clinical records used here where the ones from the first admission).
Patients without cirrhosis and presenting with ascites were excluded. When the end point evaluated was death, the period ranging from date of hospitalization admission to date of death was considered the survival period.
Data were analyzed using a statistical software program (SPSS 18). Results were expressed as mean±SD. The diferences between groups were determined by Students t test. The chi-square test was used, when appropriate, to determine the differences in proportions. The independent role of factors selected by univariate analysis was further assessed by stepwise regression analysis. Kaplan-Meier methodology was performed to analyze the survival of patients within the different groups. The log rank test was used to evaluate the statistical differences between survival curves. The Cox regression analysis was performed to analyze the Hazard risk. The statistical significance was established at a P value of less than 0.05.
Results
For interpretative purposes, patients with polymorphonuclear leucocytes ≥250 cells/mm3, either culture positive or negative, with similar clinical presentations and treated the same way, will both be considered as having SBP.
Of the 42 patients with SBP (see Table 1), 34 (81%) were male and 8 (19%) were female. SBP was diagnosed at hospital admission in 35 (83.3%) patients, in 4 of the patients infections were nosocomial and the other (n = 3) did not meet the diagnostic criteria. The mean age at admission was 57.46±13.4 years (range 36-84), with women being older (63.13±11.29 years) (p = 0.185) than men.
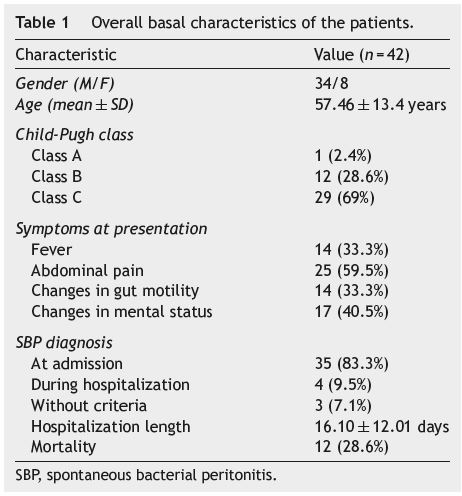
Abdominal pain, present in 25 (59.5%) patients, was the most common symptom, followed by mental status alterations (n = 17; 40.5%), fever (n = 14; 33.3%) and changes in gut motility (n = 14; 33.3%).
Twenty nine patients (69%) were classified as Child-Pugh C, 12 (28.6%) as Child-Pugh B and only one (2.4%) patient was classified as Child-Pugh A. Three patients (7.1%) were previously diagnosed with SBP, but only one of them (2.4%) was on antibiotic prophylaxis at admission. Seventeen patients (40.5%) did not have esophageal varices, and 25 (59.5%) had varices (8 [19%] with hemorrhage and 17 [40.5%] without). At hospital admission 12 patients (28.6%) were on proton pump inhibitors, 25 (59.5%) had total serum bilirubin ≥2.5 mg/dL, 21 (50%) had plasma creatinine ≥1.2 mg/dL and 13 (31%) had plasma sodium ≤130 mEq/L (see Table 2). Total serum bilirubin, plasma creatinine, plasma sodium and the presence of esophageal varices did not show a statistically significant association with a higher mortality risk.
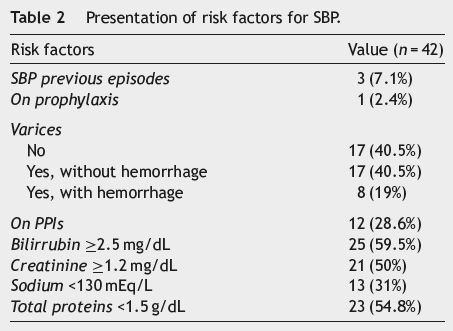
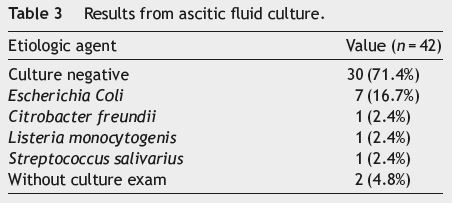
Regarding the first paracentesis done during hospitalization, 71.4% (n = 30) of the ascitic fluids analyzed were culture-negative and 4.8% (n = 2), despite having cytochemical SBP criteria, were not submitted to bacteriological testing. Escherichia coli (n = 7; 16.7%) was the pathogen most frequently isolated, with Citrobacter freundii, Listeria monocytogenis and Streptococcus salivarius being isolated once each (see Table 3). Twenty three (54.8%) patients had ascitic fluid total protein concentration <1.5 g/dL at admission; survival in these patients, however, was not statistically different from those with higher protein concentration (p = 0.612; log rank test).
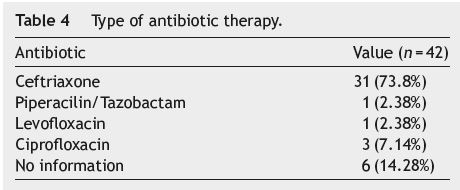
Thirty one (73.8%) patients were treated with Ceftriaxone, three (7.14%) with Ciprofloxacin, one (2.38%) with Piperacilin/Tazobactam and one (2.38%) with Levofloxacin; there was no information regarding the antibiotic regímen used in the clinical records of six (14.28%) patients. Of those on Ceftriaxone, 10 (32.25%) did not respond to the treatment and were switched to another antibiotic (see Table 4).
Of the 21 (50%) patients who repeated paracentesis during hospitalization, 19 (45.2%) had culture-negative ascitic fluid, one (2.4%) was positive for Escherichia coli and one (2.4%) for Enterococcus faecalis plus Aeromonas hydophila.
The average length of hospitalization was 16.10± 12.01 days, with men having a longer length stay (17.21±12.65 days) than women (11.38±7.70 days). Yet, this difference was not statistically significant (p = 0.221).
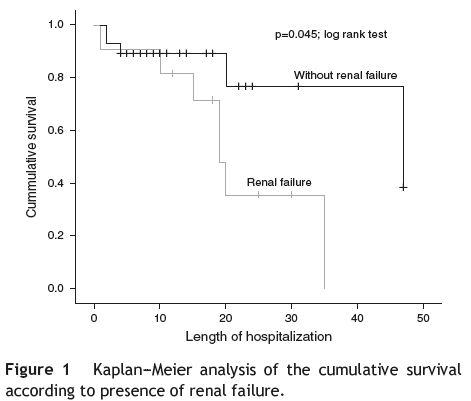
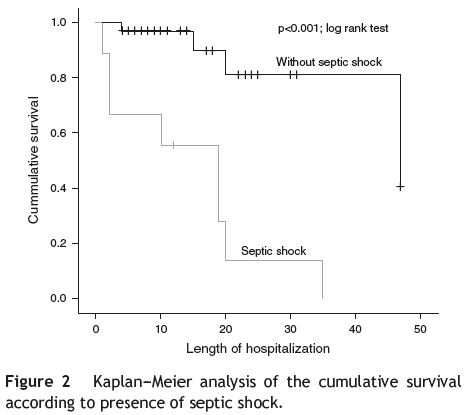
Regarding complications (see Table 5) registered during hospitalization, the presence of renal failure (RF) was associated with a higher mortality risk (OR = 8.1; p = 0.005; chi-square test), which is re-enforced by using the Cox regression (HR = 3.25; p = 0.063), suggesting a 3 times higher risk of death in these patients; there is statistical significance (p = 0.045; log rank test) when comparing the survival curves regarding the presence or absence of RF (see Fig. 1). The presence of septic shock was also associated with a higher mortality risk (OR = 54; p < 0.001; chi-square test), with a 9 times higher risk of death (HR = 9.5; p = 0.001; Cox regression); the difference between survival curves was statistically significant (p < 0.001; log rank test) (see Fig. 2).
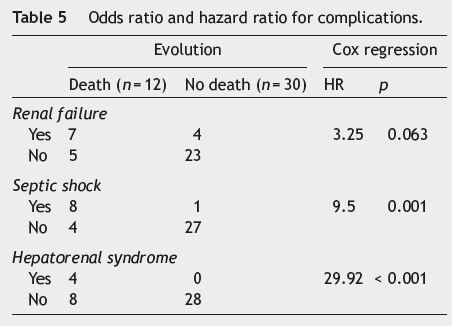
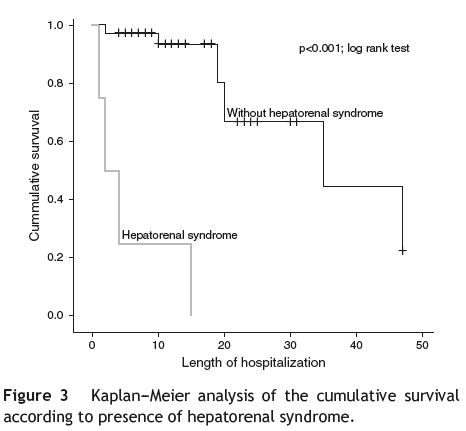
Hepatorenal syndrome was the complication associated with the higher mortality risk, a 29 times higher risk ofdeath (HR = 29.92; p < 0.001; Cox regression); the difference between survival curves was statistically significant (p < 0.001; log rank test) (see Fig. 3).
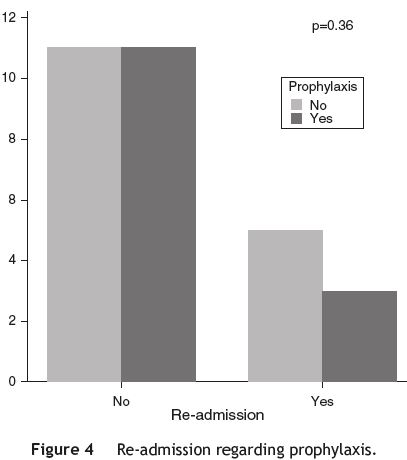
Of the 30 (71.4%) patients discharged from the hospital, 14 (46.67%) were on antibiotic prophylaxis, with 3 (21.42%) of them being later re-admitted with the same diagnosis; of the 16 (31.25%) patients discharged without prophylaxis, 5 were re-admitted. However, no statistically significant difference was found between the two groups (p = 0.36) (see Table 6 and Fig. 4).

Discussion
SBP is a common complication in patients with cirrhosisrelated ascites. With an insidious and subtle installation, its diagnosis, based on ascitic fluid cytochemical and bacteriological analysis, requires a high suspicion index.13
The aim of this study was to evaluate, in patients admitted with SBP diagnosis, the risk factors accepted in the literature as a cause for the disease and which of them influenced its prognosis.
In our series, only three of the patients had previous SBP diagnosis, with one of them being on a prophylaxis antibiotic regimen. For this reason, it was not possible to assess the effect of prophylaxis in survival.
Most patients were in an advanced phase of the disease (Child-Pugh C). Abdominal pain was the most frequente symptom at admission, although in other studies published fever was the most common symptom reported.12 However, abdominal pain can be the result of the distension caused by the ascitic fluid.
Total serum bilirubin concentration, plasma creatinine and plasma sodium levels did not alter the risk of death in a statistically significant way. In this study we retrospectivelly examined the presence of complications in association with bilirubin, creatinine and sodium levels. Further studies must include the assessment of the effect of these variables in the risk of developing complications.
The presence of hepatorenal syndrome and septic shock influenced the outcome, with those patients with hepatorenal syndrome having a twenty-nine times higher risk of death and those with septic shock having a nine times higher risk. Renal failure was also suggestively associated with death. We might say that the presence of hepatorenal syndrome and septic shock are potential predictors of mortality risk.
Ceftriaxone, suggested as the first line empiric antibiotic treatment, failed in more than 30% of SBP episodes. This is further supported by the findings of Angeloni et al.9 One may infer that it might be related with either the appearance of antibiotic resistances or with changes in etiologic agents. These results should promote further investigation aimed at identifying different treatment approaches.
Despite the latest guidelines that support the use of antibiotic prophylaxis in all patients with SBP after hospital discharge,20 in our study only 46.67% of the patients included were on prophylaxis. Nevertheless, re-admissions in this sub-group were not statistically significantly diferente from those not on prophylaxis. It is possible that no significance was found owing to a lack of statistical power based on the small number of patients included in the study.
It was not possible to evaluate in this study if SBP patients on proton pump inhibitors had a higher rate of SBP than those who were not. In further studies this should be assessed.
The fact that the study was retrospective, made it more difficult to analyze certain variables, as data was missing in some patients files. Patient search and selection was limited to patients with SBP diagnosis, based on the CDI-10 classification, by the time of discharge or death. There might have been more patients in whom this diagnosis was not done or who were not correctly codified.
References
1. Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Seminars in Liver Disease. 2008;28:26-42. [ Links ]
2. Garcia-Tsao G. Current management of the complication of cirrhosis and portal hypertension: variceal hemorrhage, ascites and spontaneous bacterial peritonitis. Gastroenterology. 2001;120:726-48. [ Links ]
3. Rimola A, Garcia-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. Journal of Hepatology. 2000;32:142-53. [ Links ]
4. Evans LT, Kim WR, Poterucha JJ, et al. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology. 2003;37:897-901. [ Links ]
5. Hoefs JC. Diagnostic paracentesis. A potent clinical tool. Gastroenterology. 1990;98:230-6. [ Links ]
6. Lata J, Stiburek O, Kopacova M. Spontaneous bacterial peritonitis: a severe complication of liver cirrhosis. World Journal of Gastroenterology. 2009;15:5505-10. [ Links ]
7. Parsi MA, Atreja A, Zein NN. Spontaneous bacterial peritonitis: recent data on incidence and treatment. Cleveland Clinic Journal of Medicine. 2004;71:569-76. [ Links ]
8. Gines P, Cardenas A, Arroyo V, et al. Management of cirrhosis and ascites. The New England Journal of Medicine. 2004;350:1646-54. [ Links ]
9. Angeloni S, Leboffe C, Parente A, et al. Efficacy of current guidelines for the treatment of spontaneous bacterial peritonites in the clinical practice. World Journal of Gastroenterology. 2008;14:2757-62. [ Links ]
10. C˘aruntu FA, Benea L. Spontaneous bacterial peritonitis: pathogenesis, diagnosis, treatment. Journal of Gastrointestinal and Liver Diseases. 2006;15:51-6. [ Links ]
11. Koulaouzidis A, Bhat S, Karagiannidis A, et al. Spontaneous bacterial peritonitis. Postgraduate Medical Journal. 2007;83:379-83. [ Links ]
12. Sheer TA, Runyon BA. Spontaneous bacterial peritonitis. Digestive Diseases. 2005;23:39-46. [ Links ]
13. Toledo C, Salmeron J, Rimola A, et al. Spontaneous bacterial peritonitis in cirrhosis: predictive factors of infection resolution and survival in patients treated with cefotaxime. Hepatology. 1993;17:251-7. [ Links ]
14. Llovet JM, Planas R, Morillas R, et al. Short-term prognosis of cirrhotics with spontaneous bacterial peritonitis: multivariate study. American Journal of Gastroenterology. 1993;88: 388-92. [ Links ]
15. Mihas AA, Toussaint J, Hsu HS, et al. Spontaneous bacterial peritonitis in cirrhosis: clinical and laboratory features, survival, and prognostic indicators. Hepatogastroenterology. 1992;39:520-2. [ Links ]
16. Follo A, Llovet JM, Navasa M, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors, and prognosis. Hepatology. 1994;10:1495-501. [ Links ]
17. Tito L, Rimola A, Gines P, et al. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology. 1988;8:27-31. [ Links ]
18. Llach J, Rimola A, Navasa M, et al. Incidence and predictive factors of first episode of spontaneous bacterial peritonitis in cirrhosis with ascites: relevance of ascitic fluid protein concentration. Hepatology. 1992;16:724-7. [ Links ]
19. Andreu M, Sola R, Sitges-Serra A, et al. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology. 1993;104:1133-8. [ Links ]
20. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, hepatorenal syndrome in cirrhosis. Journal of Hepatology. 2010;53:397-417. [ Links ]
Conflicts of interest
The authors have no conflict of interest to declare.
Acknowledgement
The authors would like to thank Rui Medeiros for the statistical analysis done.
*Corresponding author.
E-mail address: edu.gil.pinto@gmail.com (E. Rodrigues-Pinto).
Received 29 November 2011; accepted 25 April 2012













