Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Jornal Português de Gastrenterologia
Print version ISSN 0872-8178
J Port Gastrenterol. vol.20 no.6 Lisboa Dec. 2013
https://doi.org/10.1016/j.jpg.2013.05.003
ENDOSCOPIC SPOT
Endoscopic visualization of impacted bile duct stone at duodenal papilla
Aspecto endoscópico de cálculo impactado na papila de vater
Liliane C. Meireles∗, António P. Coutinho, Ana C. Lagos, Jorge M. Canena, Beatriz C. Neves
Serviço de Gastrenterologia, Centro Hospitalar Lisboa Norte - Hospital Pulido Valente, Lisboa, Portugal
*Corresponding author
Introduction
Choledocholithiasis occurs in 8-20% of patients with gallstones.1 The rate of spontaneous migration of bile duct stones through the duodenal papilla is not well known.1 An impacted bile duct stone at duodenal papilla can be associated with either cholangitis due to the complete obstruction of the bile outflow or acute pancreatitis. Endoscopic retrograde cholangiopanteatography (ERCP) has been established as the standard treatment for impacted bile duct stones. We report a case of a patient with impacted bile duct stone who underwent needle-knife fistulotomy avoiding the papillary orifice followed by standard papillotomy for the removal of the impacted stone.
Case report
A 61-year-old woman with a past history of diabetes mellitus type 2, hypertension and laparoscopic cholecystectomy performed one year ago, was admitted in the emergency room with epigastric pain, vomiting and fever. Physical examination showed jaundice and tenderness over the epigastrium. Significant laboratory results included 11,300×109/L leukocytes, C-reactive protein 6.8 mg/dL (normal value < 0.5 mg/dL), total bilirubin 3.4 mg/dL (range, 0.2-1.0), aspartate transaminase 89UI/L (range, 5-39) and alanine transaminase 206 UI/L (range, 10-49). An abdominal ultrasound showed extra-hepatic bile duct dilatation (10 mm). A CT scan showed an impacted stone at duodenal papilla. ERCP was performed and the diagnosis of na impacted bile duct stone at duodenal papilla was confirmed. The patient underwent unsuccessfully needle-knife precutpapillotomy to achieve deep cannulation. The endoscopist changed his approach to needle-knife fistulotomy which permitted standard papillotomy and ductal clearance. The fistulotomy was done in the middle of the duodenal papilla roof, 1 cm above the papillar orifice, to gain access to the bile duct. The fistula was enlarged with a standard papillotomy in order to remove the bile duct stones (Figs. 1 and 2).
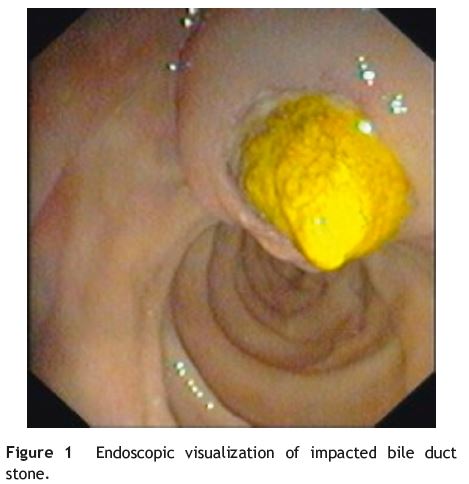
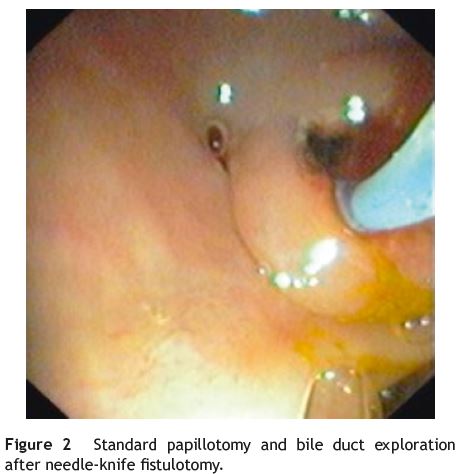
Discussion
ERCP is the standard treatment for impacted bile duct stones at duodenal papilla. However the impacted stone can lead to failure of deep cannulation with standard papillotomy and stone extraction. An endoscopic needle-knife fistulotomy can provide an artificial choledocoduodenal fistula thereby facilitating the removal of the stone.2,3 It is important after the fistulotomy a complete and large biliary sphincterotomy to permit total stone clearance and to avoid complications.
References
1. Frossard JL, Hadengue A, Amouyal G, Choury A, Marty O, Giostra E, et al. Choledocholithiasis: a prospective study of spontaneous common bile duct stone migration. Gastrointest Endosc. 2000;51:175-9. [ Links ]
2. Joo KR, Cha JM, Jung SW, Shin HP, Lee JI, Suh YJ, et al. Case review of impacted bile duct stone at duodenal papilla: detection and endoscopic treatment. Yonsei Med J. 2010;51:534-9. [ Links ]
3. Leung JW, Banez VP, Chung SC. Precut (needle knife) papillotomy for impacted common bile duct stone at the ampulla. Am J Gastroenterol. 1990;85:991-3. [ Links ]
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interest
The authors have no conflicts of interest to declare.
*Corresponding author
Correio eletrónico: lilianeenailil@gmail.com (L.C. Meireles).
Received 13 March 2013; accepted 29 May 2013













