Introduction
The diagnosis of FTP (Failure in Transfer of Passive Immunity) has been widely studied in dairy cows (Renaud, Duffield, LeBlanc, & Kelton, 2018; Topal, Batmaz, Mecitoglu, & Uzabaci, 2018; Zakian, et al., 2018). In beef cows raised in extensive farms, the research is scarce and the most adequate diagnostic and therapeutic methods are unknown, making it difficult to assess the prevalence and impacts of the condition.
In Portugal, veterinarians and farmers empirically acknowledge the importance of FTP in beef calves, but diagnostic confirmation is rarely performed and prevalence studies are limited. In beef cattle farms, the success rate at weaning is clearly conditioned by calf mortality, which in turn depends on the maternal capacity of the dam, management conditions and general health status of the herd, making the period between calving and weaning the most critical for the success of an animal management program (Pekcan, Fidanci, Yuceer, & Ozbeyaz, 2013).
In the present case report our aim was to assess the immunological status of two beef calves (less than one week old) raised under extensive conditions, presenting signs compatible with FTP, specifically biochemical parameters (GGT, globulin, and TP) and serum IgG concentration by means of an ELISA test. We also tested the feasibility of fresh frozen plasma administration in treating these calves in field conditions.
1. Theoretical framework
When the calf is born, it is almost exclusively dependent on the antibodies provided in the colostrum, since the epitheliochorial placenta of ruminants limits the transfer of immunoglobulins to the fetus in uterus (Lackshaw, 1987). Among the five main types of immunoglobulins, IgG is indispensable for the protection against viruses and bacteria, accounting for approximately 80% of the total circulating immunoglobulin in the bloodstream (Atkinson, Boyd, Sibley, & Neill, 2006). Therefore, when the cow is unable to produce or pass enough good-quality colostrum, the newborn calf fails to acquire adequate serum IgG concentration, a condition defined by Failure of Passive Immune Transfer (FPT) develops (Weaver, Tyler, VanMetre, Hostetler, & Barrington, 2000), and the newborn becomes susceptible to a series of pathologies than can have a fatal outcome, such as neonatal enteric, systemic, and respiratory diseases (Mishra, Mishra, Jyotiranjan, Behera, & Sethy, 2017). While presumptive diagnosis is usually clinical, with the presence of diarrhea and/or respiratory signs (Furman-Fratczak, Rzasa, & Stefaniak, 2011), the FTP final diagnosis is obtained by IgG quantification in the serum on the first week of life or in the colostrum of the cow, during this period (Johnson, Chancellor, Burn, & Wathes, 2017). It is generally considered that calves with a plasmatic concentration of less than 10mg/mL of IgG on the first 48 hours of life are affected by FTP (Chigerwe & Barrington, 2015).
Currently, there are several methods used to estimate IgG content in bovine colostrum and calf serum. Radial immunodiffusion (RID) estimates IgG concentration directly and currently is considered the gold standard for both colostrum and calf serum IgG quantification (Drikik, et al., 2018). However, RID is a lab-based test that requires expensive reagents and long processing times, making it unsuitable for on-farm use. Other methods may be used for laboratory evaluation of IgG concentration in colostrum and bovine plasma, such as the ELISA (Gelsinger, Smith, Jones, & Heinrichs, 2015), serum gamma-glutamyltransferase (GGT), serum protein value (TP), and the zinc sulfate turbidity test (Gelsinger, Smith, Jones, & Heinrichs, 2015; Hogan, et al., 2015). In general, the serum GGT value, ELISA tests and determining circulating globulins appear to be the most accurate assays for the laboratory detection of FTP in serum samples (Hogan, et al., 2015).
Regarding FTP treatment, it relies on the passive immunization of the affected calves, by means of plasma, serum or whole blood transfusion, which can be administered intra-peritoneal or intravenously (Chigerwe & Barrington, 2015). Colostrum replacer oral administration is also an option, depending on the age of the calf, since intestinal permeability to immunoglobulins is usually lost at 24-36h after birth (Stott, Marx, & Menefee, 1979). However, on extensive conditions it is more difficult to collect colostrum, given the character of the animals, and although IgG concentration is higher, the amount of colostrum obtained is inferior to dairy cows (McGee & Earley, 2019).
2. Methods
2.1 Study type
We hereby describe two case reports of calves presenting clinical signs compatible with FTPI and their biochemical analysis, including IgG determination by an ELISA test.
2.2 Sample
A large herd (over 300 dams) of beef cattle in the Alentejo region, in Portugal, reported a series of cases of calf deaths under 7 days of age. With the farmer’s agreement, a 4-year-old dam with no apparent signs of disease, good body condition score and that had successfully bread a calf in the previous season was selected as a blood donor. The dam was previously prepared with complete vaccination and sanitary protocols for safety guarantees. Three weeks after these protocols were completed, 8L of whole blood were collected and processed in order to obtain fresh frozen plasma (Balcomb & Foster, 2014), which was stored at -20ºC, maintaining a sample for posterior determination of biochemical parameters.
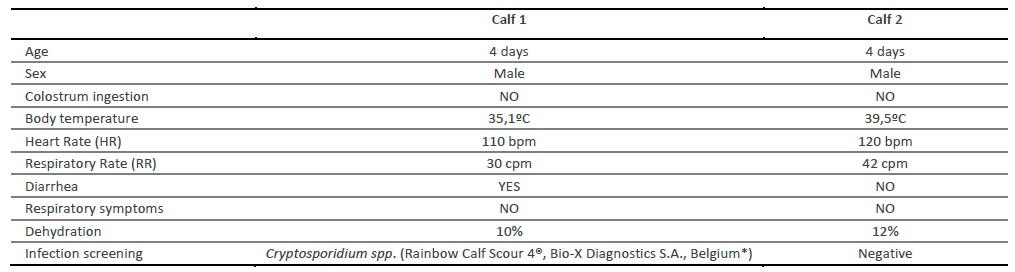
Posteriorly, two calves with an absence of colostrum ingestion and clinical signs compatible with FTP were detected, at approximately 4 days of age. A complete anamnesis, physical examination and a checklist of clinical signs were registered, confirming the clinical suspicion of FTP (Table 1). The mother of calf 1 was unable to stand and consequently to milk, due to a coxofemoral luxation, while calf 2 was unable to stand and therefore incapable of suckling by itself.
2.3 Data collection instruments and procedures
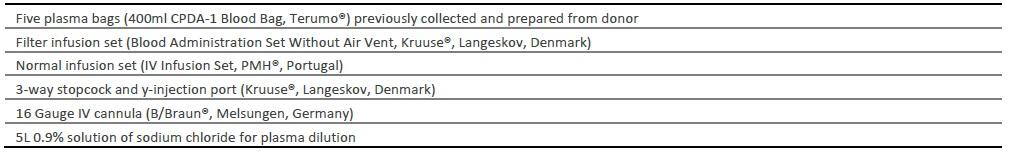
After the establishment of clinical diagnosis, the practitioner decided to initiate treatment for FTP with plasma transfusion. The two calves were treated similarly with the transfusion of approximately 2L of plasma per animal (30ml/kg) intravenously at the jugular vein, according to consulted protocols (Chigerwe & Tyler, 2010; Balcomb & Foster, 2014; Murphy, Hagey, & Chigerwe, 2014). The two animals received a simultaneous infusion of plasma and 0.9% solution of sodium chloride with an extension tube with a 3-way stopcock and y-injection port (latex-free) (Kruuse®, Langeskov, Denmark) for plasma dilution and prevention of secondary reactions (Chigerwe & Tyler, 2010; Balcomb & Foster, 2014; Murphy, Hagey, & Chigerwe, 2014). Materials and transfusion rates are described in tables 2 and 3.
Serum samples from the calves (n = 6) were collected from the jugular vein with a vacutainer needle set (Vacutest Kima®, Italy). Three collections were made per animal, the first immediately before the plasma transfusion, the second 24 hours after and the third collection 48 hours after plasma transfusion. Serum samples (2mL evacuated tubes without EDTA) were transported at 5ºC to the Agrarian School of Elvas. At arrival, the serum was harvested by centrifugation at 1000 x g at room temperature during 10 minutes and transferred to a 2mL Eppendorf tube. Serum samples were stored at -20ºC until analysis. Each sample was used to determine serum GGT, globulin and TP, as well as IgG serum concentration. For biochemical parameters, Idexx Catalyst Dx (IDEXX VetLab Station, IDEXX Europe B.V., Hoofddorp, The Netherlands) analyzer was used. A sample of 300µL of plasma was used to evaluate GGT, globulin, and TP. Additionally, TP value was also measured with a portable protein refractometer FG-301/311 (Hangzhou Chincan Trading Co., Ltd, Zhejiang, China). IgG concentrations in the serum samples were determined using a commercial direct sandwich ELISA kit Bovine IgG (cat. no. KA2046; Abnova, Taipei, Taiwan) according to the manufacturer’s instructions. The plates were read at 450 nm on a Dynex MRXII plate reader (Dynex Technologies, Virginia, USA).
3. Results
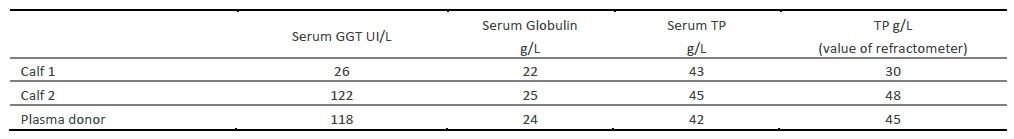
Results of biochemical parameters are described in table 4, for both calves and the donor cow.
Table 4 Biochemical parameters evaluated with Idexx Catalyst Dx (IDEXX VetLab Station, IDEXX Europe B.V., Hoofddorp, The Netherlands).
Regarding immunoglobulin concentration, the result was only determined for both calves, indicating that calf 1 and 2 presented initially a concentration of 0.7mg/ml and 1mg/ml, respectively. At 24 hours after the plasma transfusion, serum IgG concentration slightly raised to 1.8 mg/mL in calf 1 and 1.5 mg/mL in calf 2, but after 48 hours serum IgG reduced to a concentration similar to the pre-transfusion values (calf 1: 1.3 mg/mL and calf 2: 0.9 mg/mL).
Unfortunately, due to the lack of means to maintain artificial feeding of the calves, the practitioner ended up euthanizing both animals.
Discussion
The literature on passive immunity in beef calves and risk factors in the transfer failure is scarce when compared with dairy cows. McGee and co-workers (2019) have identified some aspects in which there appear to be differences between beef and dairy cattle in terms of quantity of colostrum produced, which is substantially higher in dairy cattle. While in dairy cattle there is an inverse proportionality between the amount of colostrum produced and the concentration of immunoglobulins, which define colostrum "quality", the concentration of immunoglobulins in beef cattle remains relatively constant (McGee & Earley, 2019). This could mean that, in beef cattle, the amount of colostrum ingested (and not the immunoglobulin concentration) is a critical factor for the successful transfer of immunoglobulins.
On the present work, FTP was suspected by the absence of colostrum ingestion in both clinical cases, concomitant with specific clinical signs, such as diarrhea and dehydration in calf 1 and dehydration and increase of temperature in calf 2. As has been previously mentioned, an insufficient level of colostral immunity in calves increases their susceptibility to both digestive and respiratory infections (Furman-Fratczak, Rzasa, & Stefaniak, 2011). Infectious diarrhea was actually confirmed in calf 1, with a positive diagnose for Cryptosporidium spp.
The biochemical results obtained for serum globulin and TP apparently confirm the diagnosis of FTP on both calves, since the two parameters were lower than the reference values. Although globulin levels were only slightly lower than expected - a threshold of 20-25mg/mL, TP values were clearly below the cut-off point of 52g/L (Hogan, et al., 2015). Moreover, the value of TP obtained with the biochemical analyzer was similar to the value measured by refractometry, both highly suggestive of insufficient uptake of IgG (<55g/L), confirming its usefulness for on-farm use (Tyler, et al., 1999). Although serum GGT in calf 1 was also confirmative of FTP (less than 100 UI/L) (Parish, Tyler, Besser, Gay, & Krytenberg, 1997), calf 2 presented enough GGT activity (122 UI/L), apparently compatible with colostrum ingestion. Conversely, other authors state that, on the first week of life, GGT activity should be superior to 200 UI/L (Perino, Sutherland, & Woollen, 1993), meaning that both calves would have inadequate GGT concentration. Unexpectedly, the dam’s serum globulin and TP values evaluated by both methods (automated biochemical analyzer and refractometer) were below the normal range for an adult cow. The research team failed to understand the reason for such a reduction on the biochemical parameters analyzed in an apparently healthy adult animal.
Both calves presented extremely low serum IgG concentration before the plasma transfusion treatment, largely under the threshold of 10mg/mL that identifies FTP (Chigerwe & Barrington, 2015). However, according to the results, serum IgG after 24h and 48h of the beginning of the treatment failed to increase substantially in order to confer adequate immunity to both animals. After analyzing all evaluated biochemical parameters, it is conceivable that the treatment failure could be due to the inadequate immune status of the donor cow.
The clinical decision of performing plasma transfusion instead of administering a colostrum replacement product was made according to the consulted literature (Chigerwe & Barrington, 2015), since both calves were already four days old and therefore their intestine should have become impermeable to immunoglobulins. Nevertheless, it is important to notice that, apart from immunoglobulins, there are other molecules present in colostrum and milk that regulate neonate maturation, such as microRNAs (Van Hese, Goossens, Vandaele, & Opsomer, 2020), and therefore colostrum replacement administration should not be excluded in these cases. As an alternative, plasma transfusion is a good therapeutic method to use in FTP, largely reported by authors in dairy cattle (Chigerwe & Tyler, 2010; Murphy, Hagey, & Chigerwe, 2014), but lacking conclusive results in beef cattle extensive production units, mainly due to challenging collection and transfusion conditions. Unlike dairy cattle, beef calves are usually kept with their mothers from birth to weaning. However, in beef cattle raised in extensive systems, insufficient calving follow-up may lead to difficulties in the assessment of calving difficulties and ingestion of colostrum in the critical period. On the other hand, in cases of female death or disease, abnormal maternal behavior or insufficient colostrum production, farms in extensive regimens generally do not have stored colostrum to provide to calves, hindering therapeutic alternatives (Weaver, Tyler, VanMetre, Hostetler, & Barrington, 2000).
Conclusions
The failure in the treatment of these two clinical cases reflects the importance of maintaining an adequate follow-up during the calving period, as well as performing a previous evaluation of the immunological status of donors before the plasma transfusion procedure and monitoring the calves to assure that serum IgG concentration reaches normal values. Additionally, special attention should be paid to environmental conditions, dietary and sanitary management of the herd, in order to decrease morbidity and mortality related to FTP (Weaver, Tyler, VanMetre, Hostetler, & Barrington, 2000). Finally, the existence of a fresh frozen plasma bank with selected and controlled beef cow donors would be useful in extensive production systems and would allow for advances in the investigation of FTP in beef calves