Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Obstétrica e Ginecológica Portuguesa
versión impresa ISSN 1646-5830
Acta Obstet Ginecol Port vol.10 no.4 Coimbra dic. 2016
CASO CLÍNICO/CASE REPORT
Ovarian steroid cell tumor not otherwise specified with virilizing manifestations - case report
Tumor ovárico de células esteróides sem outra especificação, com manifestações virilizantes - caso clínico
Raquel Espírito Santo*, Teresa Sabino**, Ana Agapito***
Serviço de Endocrinologia, Diabetes e Metabolismo Hospital Curry Cabral, Centro Hospitalar de Lisboa Central
*Interna do Internato Complementar de Endocrinologia e Nutrição
**Assistente Hospitalar Graduada de Endocrinologia
***Diretora do Serviço de Endocrinologia, Diabetes e Metabolismo
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Androgen-secreting tumors are present in about 0.2% of women with hyperandrogenism and more than half are malignant. Steroid cell tumors account for less than 0.1% of all ovarian tumors. The average age of presentation is 43 years, with androgenic clinical features in 56-77% of cases. The authors report a case of a 21-year-old female with virilization signs and a large ovarian mass, submitted to right salpingo-oophorectomy. Histopathologic examination revealed an ovarian steroid cell tumor not otherwise specified. A close surveillance is required attending that approximately one third of these tumors are malignant and little is known about their behavior.
Keywords: Ovarian tumor; Virilization.
Introduction
Hyperandrogenism is a term used to describe the most common clinical signs in woman with hyperandrogenemia: hirsutism, acne and alopecia. Virilization, characterized by clitoral hypertrophy, deepening of the voice, androgenic muscle development, breast atrophy, severe hirsutism, male pattern baldness, and masculine habitus, is associated with severe hyperandrogenemia.1
Androgen-secreting tumors are present in about 0.2 percent of women with hyperandrogenism and more than half are malignant2. Neoplasms may be adrenal or ovarian in origin, and must be considered in the presence of a rapid onset of hirsutism, virilization or a palpable abdominal or pelvic mass3.
Steroid cell tumors are rare ovarian sex cord-stromal tumors with malignant potential comprising less than 0.1% of all ovarian tumors4,5. A subtype called not otherwise specified (NOS) accounts for approximately one-half of all ovarian steroid cell tumors4.
The average age of presentation is 43 years, with androgenic clinical features in 56-77% of cases5,6.
Early recognition of the disease process and timely therapeutic intervention should be of foremost concern.
Case report
A 21-year-old female, on liver transplantation waiting list due to Niemann-Pick disease, was referred to an Endocrinology appointment in november/2013 for hirsutism and acne with 6 months of evolution and progressive worsening.
The menarche was at the age of 14 years, and the patient presented with amenorrhea for the last twelve months. There was no history of drug intake related with hyperandrogenism, headache, proximal muscle weakness, striae or galactorrhea.
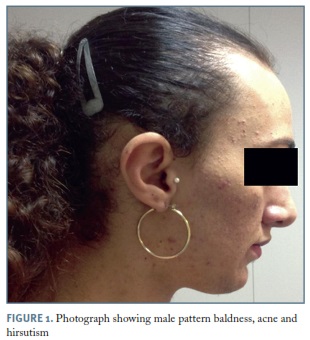
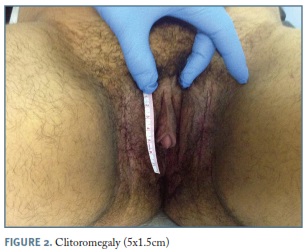
Temporal balding, acne, severe hirsutism (Figure 1), acanthosis nigricans, male muscle pattern, deepening of the voice and clitoromegaly (5x1.5cm) (Figure 2), were noticed on physical examination.
Laboratory workup revealed: total testosterone 15.89 ng/mL [0.04-0.8], free testosterone 34.74 pg/ /mL [<2.57], androstenedione >10 ng/mL [0.3-3.3], 17-hydroxiprogesterone 3.50 ng/mL [0.2-1.8], dehydroepiandrosterone sulfate 16 ug/dL [35-430], thyroid stimulating hormone 2.89 µUI/mL [0.4-4].
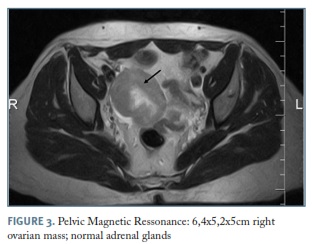
During ultrasound study in august 2013 a right adnexal mass was noted leading to a pelvic magnetic resonance, which revealed a large 6.4x5.2x5cm ovarian mass, with normal adrenal glands (Figure 3). Tumor markers were negative: cancer antigen 125 (CA 125), carbohydrate associated antigen (CA19-9), carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP).
The patient underwent surgery (18/12/2013): 20cc of ascitic fluid was evacuated and right salpingo-oophorectomy was performed. No other alterations on the abdomino-pelvic cavity were found.
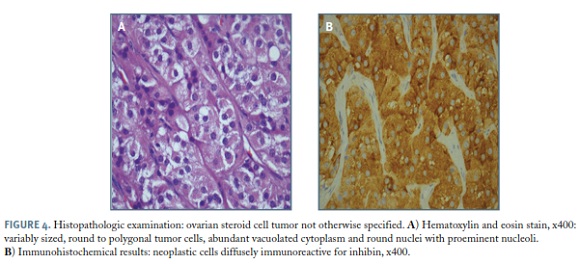
Histopathologic examination revealed: right ovary measuring 11.5x8.0x6.5cm, with a smooth and intact outer surface, totally occupied by a solid tumor. Documented absence of vascular, lymphatic or neural invasion. Presence of mild nuclear atypia, hemorrhage, four mitotic figures per 10 high-power fields. The neoplastic cells were diffusely immunoreactive for calretinin, inhibine and, focally for CAM 5.2; negative for AE1/ /AE3 and CD56 - ovarian steroid cell tumor NOS, with some features increasing the risk of malignancy: tumor diameter >7 cm, mitotic activity, hemorrhage (Figure 4).
Peritoneal fluid was negative for malignancy and the tumor was staged pT1aNxMx.
Three weeks after surgery androgen levels normalized and menses resumed: total testosterone 0.5 ng/mL, free testosterone <0.15 pg/mL, androstenedione 2.19 ng/mL, sex hormone-binding globulin 88 nmol/L (18-114).
Thrombocytopenia (platelet count 85.000x109/L [150.000-450.000]) related to her hepatic disease precluded adjuvant chemotherapy.
After surgery, progressive improvement of acne, hirsutism and temporal alopecia were noted, and androgen levels remained normal.
In 27/02/2016, the patient underwent orthotopic liver transplantation due to Niemann-Pick disease with hepatic cirrhosis complicated with portal hypertension and multifocal hepatocellular carcinoma, staged pT2(m)NxMx. Currently the patient is under immunosuppressive therapy with prednisolone 10mg id, tacrolimus 6.5mg bid and mycophenolate mofetil 500mg bid. Androgen levels remain normal 5 months after liver transplantation (total testosterone 0.07 ng/mL [0.108-0.569]).
Discussion
Adrenal or ovarian androgen-producing tumors should be suspected in women with virilization and testosterone levels greater than 100-145 ng/dL7.
Most androgen secreting ovarian tumors are sex cord stromal tumors which comprise less than 5% of all ovarian malignancies8.
Ovarian steroid cell tumors are rare neoplasms composed of cells that have typical morphological features of steroid hormone-secreting cells. The tumors may secrete several steroids and may be uncommon causes of virilization9.
Depending on the originating cell, the steroid cell tumors are categorized into three subtypes: stromal luteoma, Leydig cell tumor, and steroid cell tumor NOS4,6,7.
Steroid cell NOS represents 60% of all steroid cell ovarian tumors and are histologically identified based on absence of pathognomonic features seen in other androgen secreting ovarian tumors, such as Reinke crystals, call-exner bodies, and proeminent nucleoli5,10.
Approximately 50% of steroid cell tumors NOS exhibit symptoms of excessive androgen secretion, one third are malignant, 6% are bilateral and little is known about their behaviour5.
Occasionally, these tumors develop before puberty and in younger women, as occurred in this clinical case, but the average age reported is 43 years5,11.
Steroid cell tumors are surgically staged using criteria similar to those used in staging epithelial ovarian cancer. For women with stage IA disease (growth limited to one ovary, no ascitis, no tumor on the external surface and intact capsule), unilateral salpingo-oophorectomy is adequate. Adjuvant combination chemotherapy should be based on the histologic appearance of the tumor and on its surgical stage11. Our patient presented with some histologic features increasing the risk of malignancy, but adjuvant chemotherapy wasn’t performed due to thrombocytopenia.
As the incidence of steroid cell tumor NOS is very low, there are currently no established treatments, and the tumors are hence treated in a similar manner to stromal tumors, depending on surgical stage, histological classification and patient age.5
Due to the rarity of these tumors, little is known regarding their malignant potential and metastatic behaviour5,10,11.
Regular follow-up evaluation with subsequent monitoring of hormone levels is mandatory7,11. In this case we are performing clinical evaluation and total serum testosterone measurement each six months, which can indicate the need for immediate imaging and further evaluation.
This case is significant because of the occurrence of steroid cell tumor NOS presenting with histologic features increasing the risk of malignancy in a young patient, under immunosupressive therapy due to liver transplantation, requiring a close surveillance.
REFERENCES
1. Goodman NF, Bledsoe MB, Cobin RH, Futterweit W, Goldzieher JW, Petak SM, Smith KD, Steinberger E. American Association of Clinical Endocrinologists medical guidelines for the clinical practice for the diagnosis and treatment of hyperandrogenic disorders. Endocr Pract. 2001;7(2):120-134. [ Links ]
2. Rosenfield RL. Clinical practice. Hirsutism. N Engl J Med. 2005;353(24):2578-2588. [ Links ]
3. Hohl A, Ronsoni MF, Oliveira M. Hirsutism: diagnosis and treatment. Arq Bras Endocrinol Metabol. 2014;58(2):97-107. [ Links ]
4. Kim YT, Kim SW, Yoon BS, Kim SH, Kim JH, Kim JW, Cho NH. An ovarian steroid cell tumor causing virilization and massive ascites. Yonsei Med J. 2007;48(1):142-146. [ Links ]
5. Kim JS, Park SN, Kim BR. Recurrent ovarian steroid cell tumor, not otherwise specified managed with debulking surgery, radiofrequency ablation, and adjuvant chemotherapy. Obstet Gynecol Sci. 2014;57(6):534-538. [ Links ]
6. Jiang W, Tao X, Fang F, Zhang S, Xu C. Benign and malignant ovarian steroid cell tumors not otherwise specified: case studies, comparison, and review of the literature. J Ovarian Res. 2013;6:53. [ Links ]
7. Lambrinoudaki I, Dafnios N, Kondi-Pafiti A, Triantafyllou N, Karopoulou E, Papageorgiou A, Augoulea A, Armeni E, Creatsa M, Vlahos N. A case of postmenopausal androgen excess. Gynecol Endocrinol. 2015;31(10):760-764. [ Links ]
8. Rivera-Arkoncel ML, Pacquing-Songco D, Lantion-Ang FL. Virilising ovarian tumor in a woman with an adrenal nodule. BMJ Case Rep. 2010;2010.
9. Lin CJ, Jorge AA, Latronico AC, Marui S, Fragoso MC, Martin RM, Carvalho FM, Arnhold IJ, Mendonca BB. Origin of an ovarian steroid cell tumor causing isosexual pseudoprecocious puberty demonstrated by the expression of adrenal stereidogenic enzymes and adrenocorticotropin receptor. J Clin Endocrinol Metab. 2000;85(3):1211-1214. [ Links ]
10. Singh P, Deleon F, Anderson R. Steroid cell ovarian neoplasm, not otherwise specified: a case report and review of the literature. Case Rep Obstet Gynecol. 2012.
11. Mok JE, Sohn QS. Surgical management of steroid cell tumors of the ovary. J Gynecol Oncol. 2003;8:173-178. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Raquel Espírito Santo
E-mail: anaraquel.ces@gmail.com
Acknowledgments
The authors acknowledge Dr. Ana Pena from Department of Surgery, Dra. Ana Carvalho from Department of Pathology and Dra. Ana Morbey from Department of Hepatobiliary Surgery and Liver Transplantation, Hospital Curry Cabral - Centro Hospitalar de Lisboa Central, for the availability and indispensable cooperation in preparing this work.
Recebido em: 24/8/2016
Aceite para publicação: 26/9/2016