Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Obstétrica e Ginecológica Portuguesa
versión impresa ISSN 1646-5830
Acta Obstet Ginecol Port vol.13 no.3 Coimbra oct. 2019
ORIGINAL STUDY/ESTUDO ORIGINAL
Placenta accreta - clinical experience of a tertiary care center over 8 years
Acretismo placentar - experiência clínica ao longo de 8 anos de um centro hospitalar com cuidados terciários
Pedro Viana Pinto1, Susana Guimarães2, Ana Paula Machado3, Nuno Montenegro4
Centro Hospitalar e Universitário São João
1. Especialista de Ginecologia e Obstetrícia, Serviço de Ginecologia e Obstetrícia, Centro Hospitalar São João; Unidade de Anatomia, Departamento de Biomedicina, Faculdade de Medicina, Universidade do Porto
2. Serviço de Anatomia Patológica, Centro Hospitalar São João, National Institute of Legal Medicine and Forensic Sciences, North Branc
3. Assistente Hospitalar Graduada de Ginecologia e Obstetrí́cia do Centro Hospitalar Sã̃o Joã̃o, Porto
4. Chefe de Serviç̧o de Ginecologia e Obstetrí́cia do Centro Hospitalar de São João, EPE; Professor Catedrático da Faculdade de Medicina da Universidade do Porto; EPIUnit
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Overview and aims: Placenta Accreta Spectrum disorders are the leading cause of emergency hysterectomy in developed countries and are associated with high maternal morbidity and mortality (60% and 7%, respectively). The objective of our study was to review cases of PAS disorders occurring in the last 8 years (2010-2017) in a tertiary care centre, evaluating the forms of treatment chosen and the maternal and fetal outcomes.
Methods: Retrospective cross-sectional study using hospital databases (Obscare®, SClinico®) to identify cases of placenta accreta during the study period. Diagnosis was made based on the histological study of placentas submitted for anatomo-pathological study.
Results: During the study period, 38 cases of a PAS disorder were diagnosed in 20,910 births (1.8 / 1000), a number that is possibly underestimated. There were 8 women with a previous caesarean section and 6 had placenta previa (2 of them with previous caesarean section). There was ultrasound suspicion of a PAS disorder in 3 cases; of the 4 most severe cases (3 percretas and 1 increta), there was an ultrasound suspicion in 2. Regarding method of delivery, 21 births were by caesarean section and 16 by vaginal delivery. There was 1 case of spontaneous uterine rupture at 20 weeks' gestation with fetal loss; gestational age of birth varied between 32 and 41 weeks; 5 peri-partum hysterectomies were performed; transfusions of blood derivatives were required in 8 women. As for the newborns, there were 8 cases of prematurity and 7 hospitalisations in the Neonatal Intensive Care Unit; fetal death was observed in 2 cases.
Conclusion: This case series demonstrate the high prevalence of PAS disorders and the great morbidity associated with it. Adequate antepartum care is essential in women with riskfactors to make the diagnosis timely, thus enabling a multidisciplinary approach with better maternal and fetal outcomes.
Keywords: Placenta Accreta; Placenta Accreta Spectrum Disorders; Postpartum Haemorrhage; Postpartum Hysterectomy.
Introduction
First reviewed by Irving and Hertig in 1937, the term placenta accreta was defined as “an abnormal adherence of all or part of the placenta to the underlying uterine wall”1. Histopathologically it may be classified into three types according to the degree of invasion into the myometrium: placenta accreta vera, when the chorionic villi adhere to the myometrium; placenta increta, when the chorionic villi penetrate the myometrium; and placenta percreta, when these villi reach the uterine serosa2. Heterogeneous definitions, with misleading terms and lack of adequate pathological correlation make adequate evaluation of its prevalence, accuracy of diagnosis and outcomes difficult. In an effort to standardise, in February 2018 the International Federation of Gynecology and Obstetrics (FIGO) published consensus guidelines, proposing the classification as Placenta Accreta Spectrum disorders (PAS), involving both the abnormally adherent placenta (placenta accreta) and the invasive placenta (increta and percreta)3. This classification has also recently been endorsed by the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine and the Royal College of Obstetricians and Gynaecologists4, 5.
The most cited article regarding PAS incidence, from 2005, describes it as 1 per 533 deliveries in the American population6. Importantly, its incidence has been rising considerably in recent years, following the rise in caesarean section rates6-8. In theory, the presence of a uterine scar, with disruption of the endo/myometrial wall, whether caused by a caesarean section, probably the most important risk factor, or by a gynaecological procedure (such as curettage, myomectomy or hysteroscopy) is essential for the development of PAS9. According to a decision-analytic model, if the caesarean rates in the United States of America continued to rise, by 2020 there would be an additional 4,504 cases of PAS disorders, and consequently 130 attributable maternal deaths annually10. Another risk factor is the presence of a low-lying placenta. Placenta previa, without previous uterine surgery, is associated with a risk of 1%-5% for PAS11. According to Silver, RM. et al., the risk of PAS ranges from 2% among women with a placenta previa, to 39%-67% when it is accompanied by two or more prior caesarean deliveries12.
PAS is currently recognised as the leading cause of emergency hysterectomy in developed countries, and is associated with a maternal morbidity and mortality of 60% and 7%, respectively, mainly due to excessive blood loss13,14. The average blood loss volume at delivery is 3,000-5,000 mL11. Prenatal suspicion of PAS disorders enables planned surgery to be performed with the support of a multidisciplinary team to ensure lower maternal and fetal morbidity15-18.
In Portugal, PAS disorders are generally under-reported and unrecognised prenatally. Between 2010 and 2016, caesarean section rates in public institutions, ranged from 32.9% to 36.3%, numbers that make our country highly susceptible to PAS disorders19. We found no adequate report from any national institution regarding cases of PAS disorders, so we performed this study in order to estimate the prevalence of the PAS disorders in our institution, the adequacy of its diagnosis and maternal and fetal morbidity in the last 8 years in our centre.
Methods
We conducted a retrospective medical record review of all PAS disorder cases diagnosed in our institution (a tertiary centre) from 2010 to 2017. Cases were identified by using hospital-based electronic databases (Obscare®, Sclinico®), with cross-referencing from different databases and multiple key terms searched (low-lying placenta; manual removal of the placenta; peri-partum hysterectomy; postpartum haemorrhage). All the cases included in our study had a pathological diagnosis of a PAS disorder, the majority of them in studies of the placenta and some in hysterectomy specimens. PAS disorders were diagnosed by the presence of myometrial tissue adjacent to the chorionic villi (with immunohistochemical confirmation) with focal interruption of the decidua basal and were classified as placenta accreta, increta or percreta as previously described. The placentas were sent for pathological study according to the attending medical team’s decision. As a consequence, the diagnosis of a PAS disorder was made by combining clinical characteristics (abnormal adherence of the placenta) with confirmation by pathologic examination of the placenta.
It is important to state that the majority of the women had pregnancy surveillance outside the hospital, with most of the ultrasounds (except the first- and second-trimester ultrasounds) performed in other centres. Only women with recognised risk factors (like placenta previa) were followed up in our centre.
Medical records were analysed and data collected included demographic data (age, body mass index, parity, mode of conception); risk factors for PAS disorders (placenta previa, prior caesarean section and number, history of other uterine surgery); timing of diagnosis, antepartum and intrapartum management; gestational age at delivery; birthweight, neonatal intensive care unit (NICU) admission, Apgar scores and umbilical cord blood pH (when applicable; metabolic acidosis defined according to FIGO consensus 2015 - arterial pH < 7,00 and base deficit > 12mmol/L 20); immediate maternal morbidity; recurrence of PAS disorders. Early and delayed re-operations were defined as urgent surgical procedures performed during the first 7 days after birth/spontaneous abortion or after 7 days, respectively. Early maternal morbidity was defined as the occurrence of one or more of the following: maternal admission to the intensive care unit (ICU), transfusion of packed red blood cells, coagulopathy, ureteral or bladder wall injury or early re-operation. Late maternal morbidity was defined as the occurrence of one or more of the following: hospital re-admission within 6 weeks or delayed re-operation. Hysterectomy was classified as scheduled if the surgery was performed by a multi-disciplinary team after appropriate planning in cases with antenatal suspicion and when deemed adequate by the obstetrical team; urgent, when there was high suspicion of a PAS disorder during caesarean section and there was no significant haemorrhage; emergent when the obstetrical team was faced with severe and refractory haemorrhage and hysterectomy was considered as a final solution. During the study period there were no cases treated by a conservative approach, where the placenta is left in situ or a segmental resection of the myometrium is performed.
As a consequence of the design of the study, no statistical analysis was performed.
Results
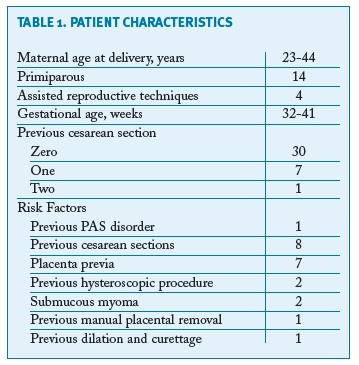
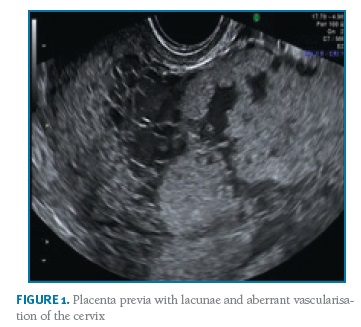
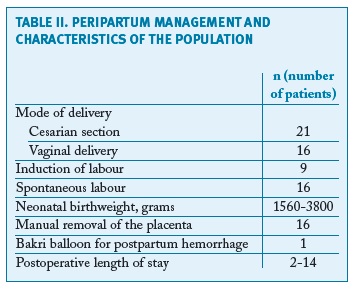
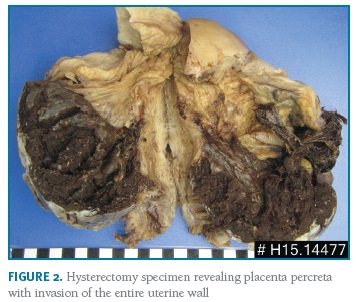
Thirty-eight cases of PAS disorders were identified between January 2010 and December 2017 in a total of 20,910 births, with a prevalence of PAS of 1.8/1000 deliveries over the 8-year study period. There were thirty-four cases of placenta accreta, one of placenta increta and three of placenta percreta. Fourteen women were primiparous, the majority (10) of them without any identifiable risk factors. Eight women had previous caesarean sections (one woman with two previous caesarean sections, the remaining with one) and seven with a low-lying placenta (three also with previous caesarean section). There were four pregnancies after assisted reproduction techniques. Two women had been submitted to a hysteroscopic procedure - one myomectomy and one septotomy. Other risk factors as well as basic characteristics of the population are summarised in Table I. All women had at least one antenatal ultrasound performed. There was no antenatal suspicion of a PAS disorder in the majority of the cases - only three cases were suspected: one case of a placenta accrete (suspicion during the third trimester), one of an increta (also suspected during the third trimester) and one of a percreta, suspected already in the first trimester and confirmed on the second trimester ultrasound (Figure 1). However, in the most serious cases (three placenta percreta and one increta), ultrasound signs suggestive of a PAS disorder were present in two (half of the cases). Only two cases had magnetic resonance imaging (MRI) performed, also confirming the ultrasound suspicion of a PAS disorder. The gestational age of birth ranged from 32-41 weeks of gestation (median of 39 weeks). There was intrapartum suspicion of a PAS disorder in 25 patients; other cases were diagnosed after pathologic examination of the placenta in cases of difficult manual removal of the placenta. The majority of births (21) were by caesarean section: twelve scheduled and nine urgent/emergent. There were 16 vaginal deliveries, all of them with manual placental removal. Table II reports peripartum characteristics of the study population. All the cases with antenatal suspicion of PAS disorders were delivered by scheduled caesarean section - attempts were made to deliver the placenta in two of them, one ending up in emergent hysterectomy. A peripartum hysterectomy after caesarean section with the placenta left in situ was performed in the other case. Five cases ended up with a hysterectomy (Figure 2).
Regarding the hysterectomies: a) in 2010 there was one case of a woman with a vaginal delivery who had only one identified risk factor (a previous dilation and curettage). After an attempt to manually remove the placenta, the patient started bleeding massively, refractory to medical attitudes and ended up with an emergent laparotomy performed with hysterectomy for bleeding control; pathology revealed a placenta percreta; b) there was one case, also in 2010, of a suspected PAS disorder during caesarean section due to placenta previa; no other risk factors were identified; an attempt to remove the placenta was made despite the intrapartum suspicion of an abnormally adherent placenta; hours after the caesarean the woman developed a coagulopathy and ended up with an emergent hysterectomy; pathology revealed a placenta percreta; c) during the year 2012, another woman had a scheduled caesarean section due to placenta previa with ultrasound suspicion of a PAS disorder; as risk factors, the woman had two hysteroscopic myomectomies and a pregnancy after assisted reproductive techniques; the placenta was forcibly removed and due to uncontrollable bleeding, and an emergent hysterectomy was performed; the patient had an iatrogenic ligation of the ureter and a laceration of the bladder, immediately corrected during surgery; pathology revealed a placenta increta; d) in 2013 there was a case of a woman with two previous caesarean sections and a placenta previa, with antenatal suspicion of a placenta percreta (by ultrasound and MRI); the patient had a scheduled caesarean hysterectomy, without any attempt of placental removal (fundal incision), with ureteral catheterisation and balloons in the common iliac arteries (inflated during surgery); surgery was performed by a multidisciplinary team - anesthesia, obstetrics, gynaecological oncology, urology and interventional radiology; deliberate cystotomy was required due to placental extension into the bladder wall; pathology revealed a placenta percreta; e) lastly, in 2015, another woman with a previous cesarean section and no other risk factors had a scheduled caesarean section; during surgery the placenta was found to be abnormally adherent and, as the patient desired definitive contraception, a hysterectomy without placental removal was decided; the surgery had no major morbidity; pathology revealed placenta accreta.
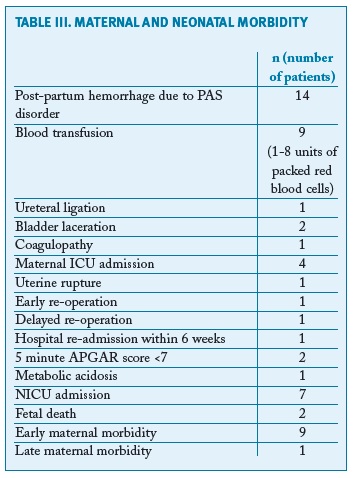
Table III summarises the maternal and neonatal morbidity in the entire cohort. There were 14 cases of postpartum haemorrhage; we found no maternal deaths due to a PAS disorder during the period of study. There was one re-admission, at the 10th day post-partum, requiring two suction curettages for complete removal of placental fragments. There were two unexpected intrauterine fetal deaths that occurred before labour: one after a uterine rupture at 20 weeks of gestation, in a woman with a previous scheduled caesarean section and a hysteroscopic septotomy. This woman had a fundal placenta, probably inserted in the scar of the septotomy, and presented in the emergency department with haemoperitoneum, secondary to uterine rupture. The patient was submitted to emergent surgery, with delivery of a non-viable fetus and uterus reconstruction. The other fetal death was secondary to a rupture of a non-diagnosed vasa previa at 32 weeks of gestation.
There were two pregnancies after a diagnosis of a placenta accreta; in one of these cases there was recurrence, with another placenta accreta diagnosed.
Discussion
During the 8-year study period, the overall prevalence of a PAS disorder in this tertiary centre was 1.8/1000 deliveries, in accordance with the published literature6,7. It is important to acknowledge that this prevalence may, in fact, be underestimated, since this study only includes placentas that were sent for pathological examination. As missed cases probably represent mild focal presentations of placenta accreta, it is arguable whether this underestimation is clinically important. Antenatal suspicion of a PAS disorder was found only in 3 out of 38 cases. This may be a consequence of the fact that the majority of our cases were mild forms of placenta accreta. Most of our cases presented without any of the classical risk factors (previous caesarean section or a placenta previa), which may have had an impact on the ultrasound evaluation of the placenta. Notwithstanding, when looking only at the abnormally invasive cases, there was an antenatal suspicion in 2 cases (half of the cases). Also, not all women had third-trimester ultrasound performed in our institution, limiting our ability to adequately predict a PAS disorder. Antenatal diagnosis of a PAS disorder is extremely important as accurate diagnosis allowing a multidisciplinary approach has been consistently shown to provide better maternal and fetal outcomes3,21-23.
Overall, the morbidity in our cohort of women with PAS disorder was high. Although most cases were histologically placenta accreta, we still had high morbidity, both maternal and fetal. In our cohort we had 14 cases of reported postpartum haemorrhage, only one managed conservatively with an intrauterine postpartum balloon; all the other cases were solved in the operating room, by artificial removal of the placenta, curettage, haemostatic sutures or hysterectomy. Nine women needed blood transfusions, with one case of placenta percreta with extension to the bladder, requiring eight units (median units transfused - 2). This demonstrates the importance of delivery of suspected cases in tertiary centres, with massive transfusion capacity. The idea of centres of excellence for the treatment of PAS disorder is now well established, strengthening the idea of multidisciplinary care and adequate conditions for treatment of this entity24, 25. Since 2014, our centre has favoured a multidisciplinary approach in order to decrease/manage haemorrhage and to prevent lesions in adjacent pelvic organs26. Our morbidity included urological lesions, a case of coagulopathy, hospital re-admission and an important case of uterine rupture at 20 weeks of gestation. In our opinion, making no attempt to remove the adherent placenta at delivery is the most important approach to reduce morbidity when there is a high suspicion of a PAS disorder.
The majority of our cases were managed conservatively. In these situations the clinician opted for manual removal of the placenta even though there was suspicion of a PAS disorder in 28 cases (25 intrapartum + 3 antepartum): in three cases it ended up in emergent hysterectomies with the associated morbidity. The extirpative technique is largely contraindicated, especially when there is a high suspicion of a PAS disorder and the decision on caesarean hysterectomy had already been taken11,27,28. Since 2014, with the creation of a specific guideline for this entity in our centre, when there is a suspicion of a PAS disorder, it is recommended no attempt be made to forcibly remove the placenta. The last hysterectomy performed during a caesarean section, in 2015, followed this recommendation and the patient ended up without significant bleeding and without the need for a blood transfusion. Some other options are reported in the literature, from the traditional approach of leaving the placenta in situ to some conservative surgical procedures29-32. In the period of study, there were no cases of conservative approaches where a segmental resection of the myometrium is performed or the placenta is left in situ. Regarding conservative surgical procedures, it is still an option limited to some centres but not generalisable to other hospitals without such surgical experience. In the future, this technique can also be an option in selected cases, as the authors report significantly lower morbidity29,31,32. Leaving the placenta in situ, with a success rate ranging from 58-78% depending on the degree of invasion30, 33, is associated with important morbidity: primary postpartum haemorrhage, infection/sepsis and need for secondary surgical procedures including emergent hysterectomy. Complete placental resorption may take up to 60 weeks, making careful follow-up essential 34. There is also concern for the risk of recurrence of a PAS disorder in women who undergo conservative management. In our series of cases, two women became pregnant after a previous PAS disorder, with recurrence in one of the cases.
Our study has some weaknesses. First, our sample size and the design of our study make it impossible to perform any statistical analyses. It is a retrospective descriptive study with the inherent limitations. Second, a standardised protocol only became available in the last years of the study, with the first cases managed according to individual clinical criteria. Third, all our cases were managed in a tertiary care centre. Adequate blood banks, 24-hour anaesthesia coverage and specialists in maternal fetal medicine and gynaecological oncology and other specialities (Urology, Surgery, Interventional Radiology) were readily available. Thus, our data may not be generalisable to other centres with fewer resources, increasing the likelihood of even greater morbidity with the management of PAS disorders in those hospitals.
However, our study has some important strengths. Thorough research was done with multiple databases in order to try to gather all the possible cases during the period of study. Our centre has all the data in computer databases providing all the information necessary to complete the study. All the cases had pathological classification, making our estimate of the prevalence as close as possible to the real value. Although the definition of accreta remains controversial, we used the most commonly agreed definition: a placenta that is abnormally adherent to the uterus and with clinical features of a PAS disorder. It is somewhat different from the proposal of the FIGO consensus, which places less emphasis on the abnormally adherent placenta, but we believe this reflects the reality and the morbidity associated with this condition as it occurs in clinical practice more adequately9. The morbidity from our cases strengthens the importance of any type of a PAS disorder.
Our series of cases report the great morbidity associated with any form of a PAS disorder. The prevalence in our centre is higher than previously assumed. It is important to be more attentive during the antenatal period in order to make the prenatal diagnosis, allowing planned multidisciplinary surgery. Our findings emphasise the importance of antenatal diagnosis for a PAS disorder and the need for increased attention to any possible risk factor in order to evaluate the placenta more adequately and optimise the care of these women. The best treatment mode will depend on the centre, the expertise of clinicians, the urgent/scheduled situation and the number of cases dealt with during a year. By changing our policy and focusing on antenatal diagnosis of a PAS disorder we believe our results will be better in the future.
Abbreviations:
FIGO - International Federation of Gynecology and Obstetrics
ICU - Intensive care unit
MRI - Magnetic resonance imaging
NICU - Neonatal intensive care unit
PAS - Placenta Accreta Spectrum disorders
REFERENCES
1. Irving FC, T. HA. A study of placenta accreta. Surgery, gynecology & obstetrics. 1937;64:178-200.
2. Garmi G, Salim R. Epidemiology, etiology, diagnosis, and management of placenta accreta. Obstet Gynecol Int. 2012; 2012:873929. [ Links ]
3. Jauniaux E, Ayres-de-Campos D, Diagnosis FPA, Management Expert Consensus P. FIGO consensus guidelines on placenta accreta spectrum disorders: Introduction. Int J Gynaecol Obstet. 2018;140:261-264.
4. Obstetric Care Consensus No. 7: Placenta Accreta Spectrum. Obstet Gynecol. 2018;132:e259-e75. [ Links ]
5. Jauniaux E, Alfirevic Z, Bhide AG, Belfort MA, Burton GJ, Collins SL, Dornan, S., Jurkovic, D.. Kayem, G., Kingdom, J., Silver, R., Sentilhes, L. Placenta Praevia and Placenta Accreta: Diagnosis and Management: Green-top Guideline No. 27a. BJOG. 2018.
6. Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005;192:1458-1461. [ Links ]
7. Higgins MF, Monteith C, Foley M, O'Herlihy C. Real increasing incidence of hysterectomy for placenta accreta following previous caesarean section. Eur J Obstet Gynecol Reprod Biol. 2013;171:54-56. [ Links ]
8. Morlando M, Sarno L, Napolitano R, Capone A, Tessitore G, Maruotti GM, Martinelli, P. Placenta accreta: incidence and risk factors in an area with a particularly high rate of cesarean section. Acta Obstet Gynecol Scand. 2013;92:457-460. [ Links ]
9. Jauniaux E, Chantraine F, Silver RM, Langhoff-Roos J, Diagnosis FPA, Management Expert Consensus P. FIGO consensus guidelines on placenta accreta spectrum disorders: Epidemiology. Int J Gynaecol Obstet. 2018;140:265-273.
10. Solheim KN, Esakoff TF, Little SE, Cheng YW, Sparks TN, Caughey AB. The effect of cesarean delivery rates on the future incidence of placenta previa, placenta accreta, and maternal mortality. J Matern Fetal Neonatal Med. 2011;24:1341-1346. [ Links ]
11. Committee opinion no. 529: placenta accreta. Obstet Gynecol. 2012;120:207-211. [ Links ]
12. Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, Moawad, A. H., Caritis, S. N., Harper, M., Wapner, R. J., Sorokin, Y., Miodovnik, M., Carpenter, M., Peaceman, A. M., O'Sullivan, M. J., Sibai, B., Langer, O., Thorp, J. M., Ramin, S. M., Mercer, B. M.. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226-1232.
13. Abuhamad A. Morbidly adherent placenta. Semin Perinatol. 2013;37:359-364. [ Links ]
14. Balayla J, Bondarenko HD. Placenta accreta and the risk of adverse maternal and neonatal outcomes. J Perinat Med. 2013;41:141-149. [ Links ]
15. Warshak CR, Ramos GA, Eskander R, Benirschke K, Saenz CC, Kelly TF, Moore, T. R., Resnik, R.. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet Gynecol. 2010;115:65-69.
16. Eller AG, Bennett MA, Sharshiner M, Masheter C, Soisson AP, Dodson M, Silver, R. M. Maternal morbidity in cases of placenta accreta managed by a multidisciplinary care team compared with standard obstetric care. Obstet Gynecol. 2011;117:331-337. [ Links ]
17. Tikkanen M, Paavonen J, Loukovaara M, Stefanovic V. Antenatal diagnosis of placenta accreta leads to reduced blood loss. Acta Obstet Gynecol Scand. 2011;90:1140-1146. [ Links ]
18. Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. The management and outcomes of placenta accreta, increta, and percreta in the UK: a population-based descriptive study. BJOG. 2014;121:62-70; [ Links ] discussion -1.
19. http://www.pordata.pt/Portugal/Cesarianas+nos+hospitais+(percentagem)-1985. [ Links ]
20. Ayres-de-Campos D, Arulkumaran S. FIGO consensus guidelines on intrapartum fetal monitoring: Physiology of fetal oxygenation and the main goals of intrapartum fetal monitoring. Int J Gynaecol Obstet. 2015;131:5-8. [ Links ]
21. Silver RM, Barbour KD. Placenta accreta spectrum: accreta, increta, and percreta. Obstet Gynecol Clin North Am. 2015;42:381-402. [ Links ]
22. Alfirevic Z, Tang AW, Collins SL, Robson SC, Palacios-Jaraquemada J, Ad-hoc International AIPEG. Pro forma for ultrasound reporting in suspected abnormally invasive placenta (AIP): an international consensus. Ultrasound Obstet Gynecol. 2016;47:276-278.
23. Collins SL, Ashcroft A, Braun T, Calda P, Langhoff-Roos J, Morel O, Stefanovic, V., Tutschek, B., Chantraine, F., European Working Group on Abnormally Invasive Placenta. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47:271-275.
24. Silver RM, Fox KA, Barton JR, Abuhamad AZ, Simhan H, Huls CK, Belfort, M. A., Wright, J. D.. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568.
25. Allen L, Jauniaux E, Hobson S, Papillon-Smith J, Belfort MA. FIGO consensus guidelines on placenta accreta spectrum disorders: Nonconservative surgical management. Int J Gynaecol Obstet. 2018;140:281-290. [ Links ]
26. Viana Pinto P, Machado AP, Loureiro T, Montenegro N. Placenta percreta - per-operatory placement of balloons in the common iliac arteries. Acta Obstet Ginecol Port. 2015;9:256-259. [ Links ]
27. Sentilhes L, Goffinet F, Kayem G. Management of placenta accreta. Acta Obstet Gynecol Scand. 2013;92:1125-1134. [ Links ]
28. Sentilhes L, Kayem G, Chandraharan E, Palacios-Jaraquemada J, Jauniaux E. FIGO consensus guidelines on placenta accreta spectrum disorders: Conservative management. Int J Gynaecol Obstet. 2018;140:291-298. [ Links ]
29. Palacios Jaraquemada JM, Pesaresi M, Nassif JC, Hermosid S. Anterior placenta percreta: surgical approach, hemostasis and uterine repair. Acta Obstet Gynecol Scand. 2004;83:738-744. [ Links ]
30. Sentilhes L, Ambroselli C, Kayem G, Provansal M, Fernandez H, Perrotin F, Winer, N. Pierre, F., Benachi, A., Dreyfus, M., Bauville, E., Mahieu-Caputo, D., Marpeau, L., Descamps, P., Goffinet, F., Bretelle, F.. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol. 2010;115:526-534.
31. Shabana A, Fawzy M, Refaie W. Conservative management of placenta percreta: a stepwise approach. Arch Gynecol Obstet. 2015;291:993-998. [ Links ]
32. Teixidor Vinas M, Belli AM, Arulkumaran S, Chandraharan E. Prevention of postpartum hemorrhage and hysterectomy in patients with morbidly adherent placenta: a cohort study comparing outcomes before and after introduction of the Triple-P procedure. Ultrasound Obstet Gynecol. 2015;46:350-355. [ Links ]
33. Matsuzaki S, Yoshino K, Endo M, Kakigano A, Takiuchi T, Kimura T. Conservative management of placenta percreta. Int J Gynaecol Obstet. 2018;140:299-306. [ Links ]
34. Sentilhes L, Kayem G, Ambroselli C, Provansal M, Fernandez H, Perrotin F, Winer, N. Pierre, F., Benachi, A., Dreyfus, M., Bauville, E., Mahieu-Caputo, D., Marpeau, L., Descamps, P., Bretelle, F., Goffinet, F.. Fertility and pregnancy outcomes following conservative treatment for placenta accreta. Hum Reprod. 2010;25:2803-2810.
Endereço para correspondência | Dirección para correspondencia | Correspondence
Pedro Viana Pinto
Centro Hospitalar São João
Portugal
E-mail: pedrovianapinto@gmail.com
Recebido em: 23/10/2018
Aceite para publicação: 18/12/2018