Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta Obstétrica e Ginecológica Portuguesa
versão impressa ISSN 1646-5830
Acta Obstet Ginecol Port vol.14 no.1 Coimbra mar. 2020
REVIEW ARTICLE/ARTIGO DE REVISÃO
A systematic approach of maternal thrombocytopenia during pregnancy
Abordagem sistemática da trombocitopenia materna durante a gravidez
Márcia Marinho1, Célia Soares1, Joana Silva2, Teresa Melo3, Claudina Carvalho4
Centro Hospitalar de Vila Nova de Gaia Espinho EPE
1 Interna de Formação Específica de Ginecologia e Obstetrícia, Serviço de Ginecologia/Obstetrícia, Centro Hospitalar GaiaEspinho
2 Assistente Hospitalar, Serviço de Ginecologia/Obstetrícia, Centro Hospitalar GaiaEspinho
3 Assistente Hospitalar, Serviço de Hematologia, Centro Hospitalar GaiaEspinho
4 Assistente Hospitalar Graduado Sénior, Serviço de Ginecologia/Obstetrícia, Centro Hospitalar GaiaEspinho
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Thrombocytopenia is the second most common haematological disorder during pregnancy, affecting 5-12% of pregnancies. Defining the cause of thrombocytopenia in pregnant women must consider not only causes related to pregnancy, but also those that occur in general population. It imposes a particular approach, because of the possible consequences to both mother and neonate. The authors review the most common causes of thrombocytopenia in pregnant and postpartum women and how to manage this condition (from the clinical history, physical examination to complementary diagnostic exams). Treatment, prognosis and monitoring and their specificities in pregnancy are also discussed in this review.
Keywords: Pregnancy; Thrombocytopenia; Diagnosis; Management; Treatment.
Introduction
Thrombocytopenia is defined as a platelet count below the 2.5th lower percentile of the normal platelet count distribution, which is <150 x 109/L in the general population.1 It is observed in 5-12% of pregnancies1-3.
During pregnancy there is an expected fall of 10% in platelet counts, which can be more pronounced in multiple gestation3-5. Thrombocytopenia is often an incidental finding and may constitute a normal physiologic response to pregnancy6,7. Increased platelet turnover, accelerated destruction of platelets passing over the placenta, decreased platelet production and dilution might explain the leftward shift present during normal pregnancy1,8,9-11. Looking at this data, some authors propose that, in pregnant women, it is reasonable to consider thrombocytopenia only when platelet counts are below 116 x 109/L as it reflects the 2.5th lower percentile of this population9.
Thrombocytopenia can be classified as follows12:
• MildPlatelet counts >100 x 109/L,
• Moderate - Platelet counts 50 -100 x 109/L, and
• SeverePlatelet counts <50 x 109/L.
Defining the cause of thrombocytopenia in pregnant women must consider causes related to pregnancy but also those that occur in general population8. It imposes a particular approach because of the possible consequences to both mother and neonate10.
This review aims to establish a systematic approach to the pregnant woman with thrombocytopenia and to describe the management of the most common causes.
Etiology
Around 75% of thrombocytopenia cases during pregnancy are due to gestational thrombocytopenia (GT), 15 to 20% to hypertensive disorders and 3-5% to immune conditions2,3,5,6,8,11,13. The cause varies according to the trimester: in the first and second, thrombocytopenia is often secondary to an immune process with immune thrombocytopenia (ITP) accounting for 75% of the cases in the first trimester5; GT is more common in the third trimester but also constitutes half of mild cases in the second trimester5; pre-eclampsia represents almost 75% of the cases of severe thrombocytopenia (< 50 x 109/L) in the third trimester6,8,14. Both pre-eclampsia, HELLP and acute fatty liver of pregnancy (AFLP) manifest predominantly in the third trimester4. Finally, thrombotic microangiopathies (TMAs) mostly occur during the latter half of gestation or post-partum6,15.
Table 1 presents the causes of thrombocytopenia in pregnancy according to their relative prevalence.
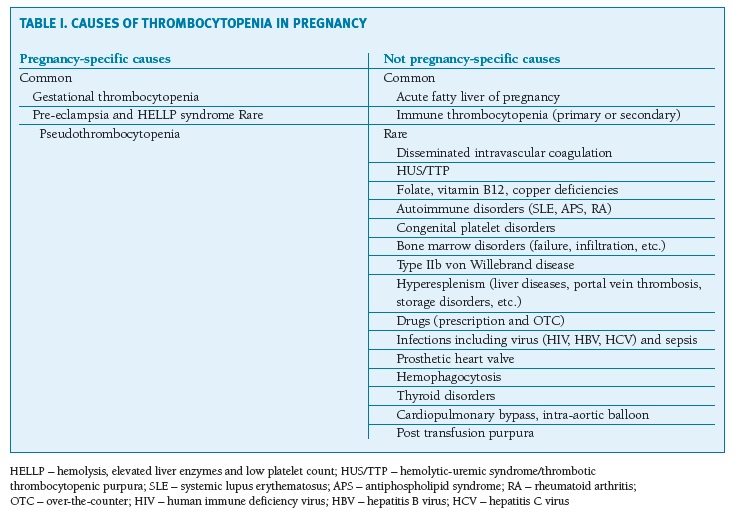
(clique para ampliar ! click to enlarge)
Pseudothrombocytopenia could be a likely cause during pregnancy9. It is secondary to platelet agglutination induced by ethylenediaminetetraacetic acid (EDTA)14,16.
GT affects 4-11% of pregnancies and is defined by thrombocytopenia (usually > 80 x 109/L), absence of symptoms, no thrombocytopenia before conception nor during early pregnancy and normalization of platelet count within 2-12 weeks postpartum.8,11 It’s an exclusion diagnosis, more frequent in the last trimester6,8,14.
ITP it’s observed in 1 in 1000-10.000 pregnant women and about two-thirds of women have the condition previously to gestation2,5,8,16,17. It is characterized by antiplatelet antibodies against GP IIb/IIIa or GP Ib/IX/V that can arise from immune deficiencies, infections (such as Helicobacter pylori) and molecular mimicry4,14.
Primary ITP is an acquired immune-mediated disorder with no obvious cause identified, while secondary ITP appears in the context of infections, rheumatologic diseases, immunodeficiencies, malignancies, drugs, vaccinations or transfusions18. When secondary to systemic lupus erythematosus (SLE) or antiphospholipid syndrome (APS), thrombocytopenia is usually less severe8.
This immune condition is typically a chronic disease with remissions and relapses18.
Often, platelet counts < 100 x 109/L occur early in gestation with progressive declining being observed8,14.
Pre-eclampsia is the most common cause of thrombocytopenia associated with TMA4,6,15 and represents 21% cases of thrombocytopenia at the time of delivery1,5. In contrast to GT and ITP, pre-eclampsia affects both platelet counts and function19.
On the other hand, HELLP syndrome (hemolysis, elevated liver enzymes and low platelet count) affects 0,5-0,9% of all pregnancies, being responsible for 12% of thrombocytopenia cases in pregnant women4,8.
AFLP, a life threatening disorder, is observed in 0,2-0,4% of pregnancies1,6,8.
Besides pre-eclampsia, HELLP and AFLP, microangiopathies of pregnancy also include thrombotic thrombocytopenic purpura (TTP), atypical hemolytic-uremic syndrome (aHUS), catastrophic APS and SLE1,15. Despite not being pregnancy-specific diseases, TTP and aHUS are TMAs that occur with increased frequency during pregnancy and postpartum period8,15.
The estimated incidence of TTP/HUS in pregnancy is 1 in 25.000.1 The time of onset is variable but it is rare in the first trimester1,5,15.
Upshaw-Schulman syndrome refers to the congenital form of TTP, caused by ADAMTS13 gene mutations15,16.
The frequency of TTP antibody is 1 in 200.000 pregnancies and 10% of women present it for the first time during pregnancy6. TTP can only be clinically apparent during pregnancy, since this state induces elevations in both von Willebrand factor (vWF) levels and uncleaved high-molecular-weight multimers and a decrease in ADAMTS138,15,16.
Atypical HUS is associated with congenital defects of the complement system alternative pathway. The condition affects 1 in 25.000 pregnancies and 10-20% of women present for the first time during gestation, probably reflecting the stress of complement activation that takes place during pregnancy6,15. Up to 80% of the cases manifest in the postpartum period and the risk is higher in subsequent pregnancies6,8.
Approach
Thrombocytopenia must always be evaluated if it is present before pregnancy or is known since childhood, if manifests in the first two trimesters, when < 80 x 109/L at any time of gestation, or when there is any known bleeding tendency9,16,19.
The typical procoagulant state of pregnancy may also explain why these women are less symptomatic5,7. Clinically significant spontaneous bleeding usually occurs with platelet counts < 10 to 20 x 109/L.10
A review of all prescription and over-the-counter drugs, especially those started 1-2 weeks prior to the development of thrombocytopenia, is essential since drug-induced thrombocytopenia is relatively common. Beta-lactam antibiotics, beta-blockers, aspirin, heparin and selective serotonin reuptake inhibitors are some examples of medications that could interfere with platelet function7,9,20 .
The nutritional history, infections, vaccinations, malignancies, alcohol ingestion, transfusions and risk factors for human immunodeficiency virus (HIV) and viral hepatitis should also be checked. Family history is important, since hereditary cases are surprisingly common7,9.
Location and severity of bleeding risk should be the focus of physical examination10. Petechiae are the hallmark of platelet-related hemorrhage9. Other bleeding signs such as ecchymosis and purpura, lymphadenopathy, splenomegaly, hepatomegaly, skeletal abnormalities or other features suggestive of inherited syndromes, hypertension, malabsorptive symptoms, neurologic abnormalities, edemas and signs of autoimmune disease should be evaluated7.
Jaundice, abdominal pain, malaise, nausea and vomiting, dyspnea and mental status alterations could suggest ALFP, while right upper quadrant or epigastric pain are more common in pre-eclampsia and HELLP syndrome6,8,14,21.
A careful review of the peripheral blood film is probably the most important procedure1,10. It has two purposes: to confirm thrombocytopenia and to exclude TMA5.
Agglutination of platelets occurs in 2% of cases9. Obtaining platelet counts in a citrate tube allows exclusion of pseudothrombocytopenia8,14,16.
Peripheral blood film may also be useful to detect abnormal platelet morphologies8.
In GT, peripheral blood smear reveals no changes in red blood cells besides a possible microcytic hypochromia, common in pregnancy4. ITP is characterized for thrombocytopenia in the absence of unusually small or giant platelets, even though the latter may be observed6,7.
There is no diagnostic testing for GT and its diagnosis relies on the detection of low platelet counts in the absence of clinical, hematological and biochemical alterations8,14. Similarly, the diagnosis of ITP is often based on a clinical history of thrombocytopenia and/or bleeding before pregnancy, family history not consistent with hereditary thrombocytopenia and exclusion of other disorders4,6-8.
Since both GT and ITP are diagnosis of exclusion, one should consider pre-pregnancy platelet counts, previous history of ITP, trimester of presentation, course of platelet count and response to treatment8.
It is impossible to differentiate ITP from severe forms of GT.5 Moreover, anti-platelet antibodies may also be present in GT, making the diagnosis harder4,22. As a general rule, the diagnosis of ITP is more consistent when platelet counts drops early in gestation and continue to fall with gestation1.
The presence of abundant schistocytes and nucleated red blood cells (along with elevation in serum LDHlactate dehydrogenase) favors the diagnosis of TMA6. Meanwhile, obtaining liver function and coagulation panel is important for the diagnosis of diffuse intravascular coagulation (DIC) and AFLP.7
A negative Coombs test excludes autoimmune hemolysis (Evans syndrome)1,7.
HELLP syndrome is characterized by thrombocytopenia, microangiopathic anemia (MAHA), serum aspartate aminotransferase > 70 IU/dL, LDH > 600 IU/dL and total bilirubin > 1.2 mg/dL. It can be presented without proteinuria or hypertension5,15.
The diagnosis of AFLP relies on documentation of impaired renal and hepatic function (impaired synthesis of clotting factors)1,5,6,20. Cholestatic liver abnormalities are common in AFLP and serum aminotransferase elevations are often 5-10 times the upper limit of normal values. The disorder can present severe thrombocytopenia (< 20 x 109/L) and DIC, which is the hallmark of AFLP, but also hypoglycemia, diabetes insipidus and reduced serum antithrombin III, the last one may be an early marker of the disease1,4,6,8.
Thrombocytopenia and microangiopathic anemia characterize both TTP and HUS6,8. In fact, although pathophysiologically different, both entities share overlapping clinical features, making differential diagnosis challenging. Acute renal failure characterizes HUS, but is less common in TTP1.
The most common form of HUS is caused by an infection with Shigatoxin producing Escherichia coli (particularly types O157:H7 and O104: H4). Despite this, aHUS is the most common form of HUS during pregnancy.
The diagnosis of aHUS is based on the presence of MAHA, thrombocytopenia with platelet counts > 50 x 109/L, progressive renal failure and sometimes evidence of ischemic organ injury, in the absence of criteria for pre-eclampsia/HELLP syndrome. Consumption of serum C3 and C4 and generation of C5b-9 complexes may be present but are not diagnostic.
The diagnosis is supported by identification of a mutation impairing alternative pathway of the complement system6.
TTP/HUS diagnosis should be considered in patients with pre-eclampsia features that don’t improve within 48-72 hours or deteriorates after delivery, especially if platelet counts drop below 20 x 109/L or in the presence of neurological dysfunction, even in the absence of family history6.
Renal dysfunction is common, so serum urea, creatinine and urine analysis are essential, like complete blood, platelet and reticulocyte counts and peripheral blood film8.
Common laboratory findings include elevated LDH, reduced haptoglobin and elevated indirect bilirubin levels14. Platelet counts < 20 x 109/L or neurological complications, along with the absence of severe renal failure, favor the diagnosis of TTP6.
The classic pentad of TTP (MAHA, thrombocytopenia, neurologic dysfunction, fever and renal injury) is present only in 40% of patients16.
Some features may help differentiating TTP from HELLP syndrome (favoring the first one): severe hemolysis (LDH/AST ratio > 22 in the third trimester) and thrombocytopenia, presence of fever or neurologic dysfunction, lower frequency of abdominal pain, hypertension and proteinuria, lower increase in transaminase levels, absence of coagulopathy, and persistence of symptoms for more than 72 hours after delivery16.
The diagnosis of TTP is based on the determination of ADAMTS13 levels < 10% of normal1,6,8.
Bone marrow examination is rarely necessary7,8. Current guidelines recommend it when peripheral blood smear abnormalities is seen or the patient is refractory to treatment18.
Flow-chart 1 presents the algorithm evaluation for thrombocytopenia in pregnant women.
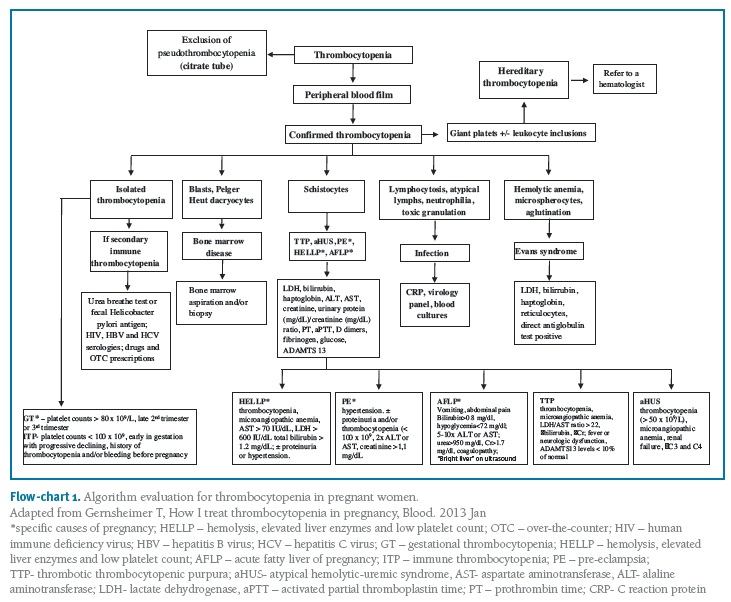
(clique para ampliar ! click to enlarge)
Those cases with a family history of bleeding should be referred to a hematologist.
Treatment
The antenatal management aims to achieve a “safe” level to both mother and fetus, usually > 20 to 30 x 109/L, since spontaneous bleeding can occur when platelet counts are bellow these values5.
Treatment of thrombocytopenia is usually directed to its specific cause.
Platelet transfusions must be reserved for life-threatening situations and only after IVIg (for example bleeding or platelet counts < 30 x 109/L).; corticosteroids may also be used if the cause is unknown and there are no contraindications9.
General measures include avoidance of aspirin (but only if there is a high risk of bleeding), anti-inflammatory drugs and intramuscular injections5,6,8.
In GT, usually no specific measures are necessary besides periodic monitoring8.
Treatment of ITP includes high-dose IVIg (1 g/kg for 2-5 days) and/or corticosteroids (typically prednisone 0,51 mg/Kg/day gradually titrated to the lowest effective dose once response is achieved)1,6-8. Low-dose steroids are generally used first and the response time is around 3-7 days5,23.
ITP treatment should be considered in following scenarios:
• -in the presence of bleeding in the first or second trimester1
• -platelet counts < 20 - 30 x 109/L in the absence of bleeding in the first or second trimesters14
• -platelet counts < 50 x 109/L in the third trimester14
• -for procedures and delivery (when platelet counts < 20 - 30 x 109/L for vaginal deliveries and < 50 x 109/L for cesarean section)6.
Therefore, most patients do not require treatment until delivery is imminent1. There is a rapid onset of action for IVIg, but the effect is usually transient (1-4 weeks)4.
Splenectomy (preferably in the second trimester for refractory cases), immunosuppressant agents such as azathioprine, high dose methylprednisone pulses and anti-D immunoglobulin are second-line agents1,6,13,14. Aminocaproic acid could also be considered before and after delivery in cases of severe ITP.6 Human recombinant thrombopoietin and thrombopoietin receptor agonists, cyclosporine A, dapsone, Campath-1H and rituximab have been used in anecdotal reports1,9,10.
The management of pre-eclampsia/eclampsia, HELLP syndrome and AFLP is essentially supportive until definitive treatment (delivery)1,6,8.
Management of TTP/aHUS includes plasma exchange as the most effective therapy, dialysis in case of renal failure and management of the underlying disorders6,8. Plasmapheresis for acquired TTP, plasma infusion for congenital TTP and complement inhibitor with eculizumab for aHUS are treatment options, but steroids, immunosuppressant agents and rituximab lack evidence for their systematic use6.
Plasmapheresis should be started once the diagnosis of TMA is established and continued until the TTP is excluded6. Although a trial of plasma exchange should be undertaken in aHUS, the response is poor; eculizumab appears to be safe in pregnancy5. Virology screening is mandatory before plasma exchange and the latter is continued until platelet count is > 150 x 109/L for at least three days and serum LDH normalizes1.
Aspirin and low molecular weight heparin at prophylactic doses may be started once platelet count is > 50 x 109/L1.
Delivery may be indicated in the absence of response to plasma exchange1,8.
Drug-induced thrombocytopenia must be considered and usually resolves in 5-10 days after drug withdrawal10.
The management of DIC implies correcting the underlying precipitant, but fresh frozen plasma, platelet transfusion, cryoprecipitate or fibrinogen concentrate may be necessary14.
Prognosis
Thrombocytopenia could be associated with poorer maternal (cesarean section incision hemorrhage) and fetal outcomes (neonatal thrombocytopenia)24,25.
Nevertheless, common practices are universal to maternal thrombocytopenia: bleeding is rare with platelet counts > 50 x 109/L; platelet counts should be higher than 75-80 x 109/L for performing an epidural, but the cut-off should be individualized4,5,13. Moreover, there is no evidence that cesarean section is safer for the fetus5.
Even though some cases of GT may affect the ability to offer epidural anesthesia, it does not affect maternal or fetal outcomes8,14,26. GT isn’t a contraindication to neuraxial anesthesia and vaginal delivery is preferred4,19. Fetal thrombocytopenia is rare (< 2%) and GT is expected to resolve within a few days to 1-2 months after delivery6,7. A small subset of women develop a significant thrombocytopenia and a reduction in antithrombin III, which might account for a higher risk of recurrence in subsequent pregnancies6.
Most patients with ITP improve with no therapy or first-line treatment.18 Maternal and fetal outcomes are usually favorable and pregnancy has not been shown to worsen its course, although ITP could increase the risk of post-partum hemorrhage in the first 24-48 hours6,8,16,19.
Maternal clinical characteristics are unable to predict neonatal thrombocytopenia, except for the existence of a thrombocytopenic sibling and (less likely) the maternal post-splenectomy status, which instead correlates with a higher risk of intracranial hemorrhage in the neonate5,6,8,27. Umbilical cord platelet counts should be determined at delivery8,14. Until a safe platelet count is achived, intramuscular administration of vitamin K should be avoided7.
Neonatal mortality is less than 1%7. Major hemorrhagic complications occur in 3% and intracranial bleeding in 0-1,5% of infants, so a cranial ultrasound may be indicated when platelet counts < 50 x 109/L or in the presence of neurologic impairment6,8,11,14.
Neonatal cases of bleeding are not usually caused by trauma7. Therefore, the mode of delivery should be determined by obstetric indication4,7. Due to the risk of neonatal bleeding, forceps are the preferred instrument and special care should be taken when performing assisted vaginal delivery6,14.
Pre-eclampsia, HELLP syndrome and AFLP usually resolve within a few days after delivery, even though analytical changes may be observed for additional 24-48 hours8,28-30.
In TTP/HUS, most deaths occur within 24 hours of clinical presentation in untreated patients1. Fetal loss due to placental ischemia is frequent in the first two trimesters but early treatment with plasma exchange may allow successful continuation of pregnancy1,6.
The risk of recurrence is near 100% for congenital TTP and 20-50% in acquired TTP, so plasma infusions should be instituted as early as the first trimester when plasma levels < 5-10% of normal1,6,8. In atypical HUS, the risk of fetal loss (10-20%) is probably related to the presence of microthrombi in maternal renal vasculature, while the risk of recurrence is about 10-30%6.
Monitoring
The monitoring frequency of platelet counts should take in account clinical reasoning and the specific cause1. Full blood count is usually checked monthly until the third trimester and then every two weeks and weekly as delivery approaches5. When there is a high certainty for the diagnosis of GT, platelet counts may be checked at each consultation and again 1-3 months after delivery to confirm the diagnosis1.
In suspected ITP, monitoring of platelet counts should occur monthly in the first and second trimesters, every two weeks after 28 weeks of gestation and weekly after 36 weeks4.
Monitoring for neonatal ITP is necessary for several days or months after delivery1,4.
There is a need for intensive postpartum monitoring in HELLP syndrome because laboratory abnormalities often worsen 1-2 days after delivery, while platelet counts are expected to rise by the fourth day1.
In acquired TTP, monthly monitoring of platelet counts and ADAMTS13 activity in subsequent pregnancies should take place, even in the presence of normal ADAMTS13 levels5,6.
Conclusion
Although GT is the leading cause of thrombocytopenia in pregnant woman and it is not associated with unfavorable outcomes, the distinction from ITP, hypertensive and microangiopathic disorders is important since these entities may represent significant maternal and neonatal morbidity9. During the last weeks of pregnancy, the predominance of GT as a leading cause of maternal thrombocytopenia is notorious, but one should not forget that thrombocytopenia may represent a clinical manifestation of HELLP syndrome or AFLP1. Despite representing only 3% of all cases of thrombocytopenia in pregnancy, ITP is the most common cause of severe thrombocytopenia during the first and second trimesters6.
The differential diagnosis (Table II) may be challenging in some clinical pictures: (1) both GT and ITP may resolve in the postpartum and recur in subsequent pregnancies and severe GT is sometimes impossible to differentiate from ITP, (2) the distinction between AFLP and HELLP is not always straightforward (3) there is a considerable clinical overlap between severe pre-eclampsia/HELLP and TMAs, especially latter in pregnancy and post-partum period, and both can occur simultaneously since TTP confers an increased risk of pre-eclampsia, which could delay the diagnosis.
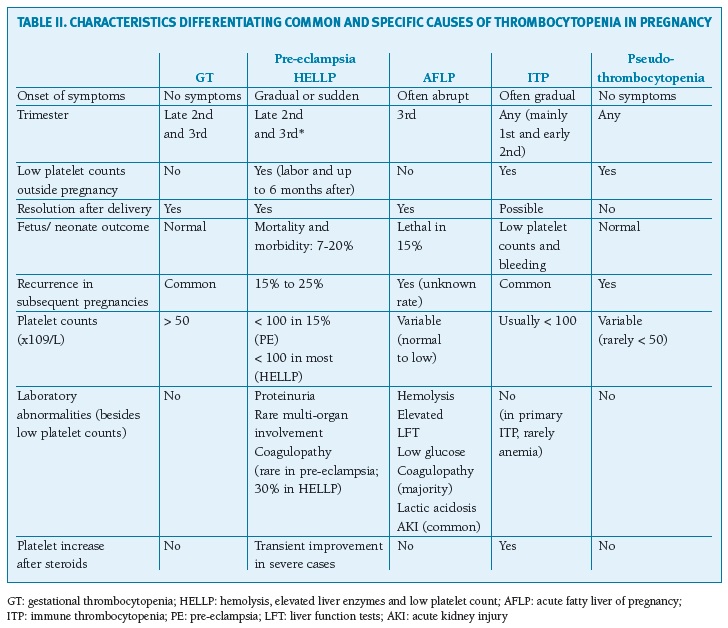
(clique para ampliar ! click to enlarge)
List of common abbreviations
AFLPacute fatty liver of pregnancy
aHUSatypical hemolytic-uremic syndrome
APS - antiphospholipid syndrome
ASTserum aspartate aminotransferase
CMV - cytomegalovirus
DICdiffuse intravascular coagulation
EBV - EpsteinBarr virus
EDTAethylenediaminetetraacetic acid
GTgestational thrombocytopenia
HBV - hepatitis B virus
HCV - hepatitis C virus
HIV - human immune deficiency virus
HELLP - hemolysis, elevated liver enzymes and low platelet count
HUS - hemolytic-uremic syndrome
ITPimmune thrombocytopenia
LDH - lactate dehydrogenase
MAHAmicroangiopathic anemia
SLE - systemic lupus erythematosus
TMAthrombotic microangiopathies
TTPthrombotic thrombocytopenic purpura
REFERENCES
1. Gernsheimer, T., A.H. James, and R. Stasi, How I treat thrombocytopenia in pregnancy. Blood, 2013. 121(1): p. 38-47
2. Reese, J.A., et al., Platelet counts in women with normal pregnancies: A systematic review. Am J Hematol, 2017. 92(11): p. 1224-1232
3. Reese, J.A., et al., Platelet Counts during Pregnancy. N Engl J Med, 2018. 379(1): p. 32-43
4. Bockenstedt, P.L., Thrombocytopenia in pregnancy. Hematol Oncol Clin North Am, 2011. 25(2): p. 293-310, vii-viii [ Links ]
5. Myers, B., Diagnosis and management of maternal thrombocytopenia in pregnancy. Br J Haematol, 2012. 158(1): p. 3-15
6. Cines, D.B. and L.D. Levine, Thrombocytopenia in pregnancy. Hematology Am Soc Hematol Educ Program, 2017. 2017(1): p. 144-151
7. Gernsheimer, T.B., Thrombocytopenia in pregnancy: is this immune thrombocytopenia or...? Hematology Am Soc Hematol Educ Program, 2012. 2012: p. 198-202
8. Palta, A. and P. Dhiman, Thrombocytopenia in pregnancy. J Obstet Gynaecol, 2016. 36(2): p. 146-52
9. Izak, M. and J.B. Bussel, Management of thrombocytopenia. F1000Prime Rep, 2014. 6: p. 45
10. Stasi, R., How to approach thrombocytopenia. Hematology Am Soc Hematol Educ Program, 2012. 2012: p. 191-7
11. American College of, O. and B.-O. Gynecologists' Committee on Practice, Practice Bulletin No. 207: Thrombocytopenia in Pregnancy. Obstet Gynecol, 2019 [ Links ]
12. Siddall, J., Maternity Guidelines - Thrombocytopenia in Pregnancy 2017 [ Links ]
13. Robinson, S., K. Longmuir, and S. Pavord, Haematology of pregnancy. Medicine, 2017. 45(4): p. 251-255
14. Yan, M., A.K. Malinowski, and N. Shehata, Thrombocytopenic syndromes in pregnancy. Obstetric medicine, 2016. 9(1): p. 15-20
15. Gupta, M., B. Feinberg, and R. Burwick, Thrombotic Microangiopathies of Pregnancy: Differential Diagnosis. Pregnancy hypertension, 2018 [ Links ]
16. Bergmann, F. and W. Rath, The Differential Diagnosis of Thrombocytopenia in Pregnancy. Dtsch Arztebl Int, 2015. 112(47): p. 795-802
17. Care, A., et al., Severe primary autoimmune thrombocytopenia in pregnancy: a national cohort study. BJOG, 2018. 125(5): p. 604-612
18. Ma, I. and A.T. Sandhu, Immune Thrombocytopenia. Hospital Medicine Clinics, 2017. 6(1): p. 53-66
19. Forest, D.J. and S. Segal, Update on Thrombocytopenia in Pregnancy and Neuraxial Anesthesia. Current Anesthesiology Reports, 2017. 7(1): p. 111-118
20. Visentin, G.P. and C.Y. Liu, Drug-induced thrombocytopenia. Hematology/oncology clinics of North America, 2007. 21(4): p. 685-696
21. Liu, J., Ghaziani, T. et al, Acute fatty liver disease of pregnancy: updates in pathogenesis, diagnosis, and management. American Journal of Gastroenterology, 2017. 112(6), 838-846. [ Links ]
22. Goldman, B.G., et al., The presentation and management of platelet disorders in pregnancy. Eur J Haematol, 2018. 100(6): p. 560-566
23. Xu, X., et al., Evaluation of glucocorticoid compared with immunoglobulin therapy of severe immune thrombocytopenia during pregnancy: Response rate and complication. Am J Reprod Immunol, 2018: p. e13000
24. Larroca, S.G., et al., Platelet Count in First Trimester of Pregnancy as a Predictor of Perinatal Outcome. Open Access Maced J Med Sci, 2017. 5(1): p. 27-32
25. Dwivedi, P., et al., Fetomaternal outcome in pregnancy with severe thrombocytopenia. Eur Rev Med Pharmacol Sci, 2012. 16(11): p. 1563-6
26. Ruggeri, M., et al., Gestational thrombocytopenia: a prospective study. Haematologica, 1997. 82(3): p. 341-342
27. Care, A., et al., Severe Primary Autoimmune Thrombocytopenia (ITP) in Pregnancy: a national cohort study. BJOG, 2018. 125(5): p. 604-612
28. Brown, M., et al., Can we predict recurrence of pre-eclampsia or gestational hypertension? BJOG: An International Journal of Obstetrics & Gynaecology, 2007. 114(8): p. 984-993
29. Surapaneni, T., V.P. Bada, and C.P.K. Nirmalan, Risk for recurrence of pre-eclampsia in the subsequent pregnancy. Journal of clinical and diagnostic research: JCDR, 2013. 7(12): p. 2889
30. Sullivan, C.A., et al., The recurrence risk of the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP) in subsequent gestations. American Journal of Obstetrics & Gynecology, 1994. 171(4): p. 940-943
Endereço para correspondência | Dirección para correspondencia | Correspondence
Marcia Marinho
Centro Hospitalar de Vila Nova de Gaia Espinho EPE
Porto, Portugal
E-Mail: marciasilvamarinho@gmail.com
Recebido em: 05/09/2019
Aceite para publicação: 24/03/2020














