Introduction
On monochorionic twin pregnancy a single placenta is shared and vascular anastomoses on the fetal surface connect the blood circulation of both twins. (1 One of the unique pathologies of monochorionic twins is twin-to-twin transfusion syndrome (TTTS), where an unequal distribution of blood between the two fetuses is significant enough for twin oligohydramnios-polyhydramnios sequence (TOPS) to happen. (2), (3 Another recently described pathology, twin anemia-polycythemia sequence (TAPS), also results from unequal blood distribution caused by slow blood transfusion between them. (4 Occurring spontaneously or after laser treatment for TTTS, TAPS brings us clinical challenges on diagnosis and management. This review aims to summarize the research made on the last 7 years on TAPS, raising awareness and enlightenment on this under-recognized pathology.
Material and methods
A search in Pubmed using the terms “Twin anemia-polycythemia” restricted to the last five years was conducted in spring summer 2018 (concerning articles between august 2013 and august 2018) and newly updated in march 2020 for a total span of seven years of literature, with a total of 135 articles found within the languages spoken by the authors (Portuguese, English, Spanish, German or French). 6 articles were excluded because they did not contain specific information on TAPS, thus remaining 129 articles. After careful reading, sections of 58 articles were selected to be quoted in this narrative literature review.
The monochorionic placenta
Almost all the monochorionic (MC) twins have vascular anastomoses between the two fetal circulations5), (6, while dichorionic twins share no anastomosis. (6 There are three types of anastomosis - artery-to-vein (AV), artery-to-artery (AA), and, less commonly6, vein-to-vein (VV).
AV anastomoses are by definition unidirectional, with the blood flowing, deeply7, from the artery of one twin to the vein of the other. (1), (8 Almost all of monochorionic placentas have multiple AV anastomoses. (9
AA and VV anastomoses are superficial8 and the blood flow can be bidirectional5. AA anastomoses are considered protective against the development of TTTS and TAPS as they counterbalance the flow of the unidirectional AV anastomoses, but the presence of an AA anastomosis does not exclude the possibility of TAPS or TTTS. (9 A disproportion in the ratio10 or diameter9 between the AV and the AA anastomoses is thought to be a key component in the hemodynamic imbalance that ultimately causes these pathologies. The role of VV anastomoses is unclear. (5
Twin-to-twin transfusion syndrome (ttts)
TTTS is a syndrome that happens in about 10-15%3), (10 of monochorionic pregnancies. (1 The diagnosis of TTTS is based on the presence of twin oligohydramnios-polyhydramnios sequence (TOPS). To diagnose TTTS, two ultrasound criteria are required: the pregnancy is diagnosed as monochorionic twin pregnancy (ideally with a scan carried out in the first trimester showing a T-sign, a monochorioninc characteristic sign of the 2 opposing amnions at the base of the separating membrane) (2), (11, and a combination of oligohydramnios in one twin’s amniotic cavity (the “donor”) with polyhydramnios in the other twin’s amniotic cavity (the “recipient”) - the so called TOPS. (1), (11 Oligohydramnios is defined as a deepest vertical pocket (DVP) <2 cm in the donor twin. Polyhydramnios is defined as a DVP of >8 cm, although in Europe a cut-off of DVP of >10 cm is traditionally used after 20 weeks. (1), (11
Twin anemia-polycythemia sequence (taps)
Twin anemia-polycythemia sequence (TAPS) was only first described, as its post-laser form, in 2006, while the spontaneous form of TAPS was first described in 2007. (4 Its major feature is the discrepancy of the hemoglobin values between the anemic fetus (the donor) and the polycythemic one (the recipient) caused by slow blood transfusion - estimated to be around 5 ml to 15 ml per day4 - due to AV anastomoses not counterbalanced by a large enough AA anastomosis.
Spontaneous TAPS has been said to occur in between 3-5% of monochorionic diamniotic pregnancies4), (11), (12, though one single-center study recently reported a smaller incidence of 1,6%.13 It has also been reported twice in very unusual dichorionic pregnancies. (14), (15
However, TAPS is more frequent after laser-treated TTTS, with an incidence of 2-16%4), (16. Even with optimal surgical technique, some patent intertwin-anastomoses might remain present after laser ablation. (8 Its reported frequency varies widely8; most of the residual anastomoses are small and peripherally located. (8), (9), (17 It is important to notice that not all residual anastomoses after TTTS laser surgery cause pathology. (18 This post-laser TAPS usually occurs acutely within one or two weeks after the surgery3, but much later cases have been reported. (19
Diagnosis
Prenatal
Prenatal recognition of TAPS is essential for the management of these pregnancies. The absence of TOPS is an essential element in the diagnosis of TAPS, as TOPS is only an hallmark for TTTS. (4), (20 Besides that, prenatal diagnosis is based on the Doppler ultrasound middle cerebral artery-peak systolic velocity (MCA-PSV). (4 During the past decade, different MCA-PSV values for the diagnosis of TAPS have been proposed. (4 An increased MCA-PSV has been well established for the diagnosis of fetal anemia, with a MCA-PSV >1.5 multiples of the median (MoM) being reported as very sensitive21, but the evidence supporting a decrease of MCA-PSV on fetal polycythemia, however, is much more scarce. (21
A study22 concluded that, although there was a correlation between MCA-PSV and anemia, MCA-PSV was similar among polycythemic and normal fetuses and that even severely polycythemic fetuses might have a normal MCA-PSV, and suggested that the difference in intertwin MCA-PSV (delta MCA-PSV) might serve as a reliable tool for a prenatal diagnosis of TAPS, as it was positively correlated with the intertwin hematocrit difference. Another recent studies23), (24, corroborated these findings, helping establishing delta MCA-PSV >0.5 MoM as having higher diagnostic accuracy for TAPS.
Another proposed clue for the prenatal suspicion and diagnosis of TAPS is placental dichotomy, it is, one part of the placenta, usually the one from the anemic twin, being reported as hyperechogenic and the other portion as hypoechogenic. (25), (26
Postnatal
The postnatal criteria is mainly based on hematological criteria at birth. (27 Currently an inter-twin hemoglobin difference >8 g/dL is the base of the postnatal diagnosis. (11), (12), (27 As a large Hb difference (>8 g/dL) at birth is also detected in case of acute peripartum TTTS at least one of two additional criteria must be fulfilled: (4), (27 An inter-twin reticulocyte count ratio (measured by dividing the reticulocyte count of the donor by the reticulocyte count of the recipient). (4 over 1.7 is pathognomonic for TAPS (as a result of increased erythropoiesis in the donor due to chronic anemia). (4; the other established postnatal criterion is the presence of small residual anastomoses, of less than 1 mm of diameter4 (as acute peripartum TTTS, in contrast to TAPS, is thought to be based on large placental AA or VV, allowing a large amount of blood to flow during the birth). (4
The common find of pale parenchyma on the maternal side of the placenta portion of the anemic twin and dark red parenchyma on the polycythemic twin portion might become an additional criterion for postnatal diagnosis in the future. (28), (29
TAPS VS TTTS
TTTS and TAPS have been described as mutually exclusive diagnoses30, as a diagnosis of TTTS is given to any monochorionic pregnancy with TOPS11, and TAPS diagnosis implies the absence of TOPS. (4), (20
However, this point of view has not been unanimous in our literature review, with some studies mentioning “TAPS combined with TTTS” (31 or “TTTS cases with secondary TAPS” (10, “coexisting TAPS” (32 or “presence of TAPS with TTTS” (33. On the other hand, it is clear that there are some cases in the cross between TAPS and TTTS, and that there is a continuum between these conditions20. A study aimed to survey cases of TTTS with superimposed twin anemia-polycythemia (AP) (30 and found that 2.4% (9/369) of TTTS patients had TTTS+AP (based on discordance MCA-PSV values). Such a small fraction of TTTS patients having MCA-PSV suggestive of anemia/polycythemia supports the hypothesis that the mechanisms responsible for the amniotic fluid discordances seen in TTTS may be different than the mechanisms responsible for the large fetal hemoglobin differences seen in TAPS. (30
It is interesting to note that amniotic fluid discordance(AFD) >3 cm (and that does not fulfil the TTTS criteria) has been shown to increase the risk for TAPS. (34
TAPS VS ACUTE TTTS
Acute peripartum TTTS is a rare form of twin-to-twin transfusion - estimated to occur in about 1.2%12 to 2.5% of all MC twins35 - thought to be due to acute shifts of blood between the two fetuses during delivery. (27
The physiopathology is not clear and based mainly on case reports and small series, but it is thought that acute peripartum TTTS may be due to acute shifts of relatively large volumes of blood from one twin to the other due to blood pressure differences following uterine contractions or changes in fetal positions, (27), (35 which is likely only possible through large AA or VV superficial anastomoses27
To reach the diagnosis of acute peripartum TTTS in a twin pair with large hemoglobin difference at birth, the presence of chronic TTTS and TAPS must be ruled out. (35 A key factor is that in acute TTTS, unlike in TAPS, the reticulocyte counts are not elevated. (27 Colour difference between the two portions of the placenta also seems to be notably stronger in TAPS. (36 The distinction between acute peripartum TTTS and TAPS is important as acute TTTS donors suffer from acute blood loss (resulting in severe anemia and hypovolemic shock) (27), (36 and may therefore need acute blood transfusions (and volume expanders) within the first few hours after birth27), (36, while TAPS donors (as TAPS is a chronic process) would benefit from a more conservative therapeutic approach with slower blood transfusion (or even no blood transfusion at all) to prevent hemodynamic complications. (36 Therefore, a quick distinction between acute peripartum TTTS and TAPS within one hour of birth could be helpful. (36
The differences between these three entities are summed up on Table I.
TABLE I Differences between TTTS, TAPS AND ACUTE TTTS. Adapted from Hematological disorders at birth in complicated monochorionic twins” by L.Verbeek et al.
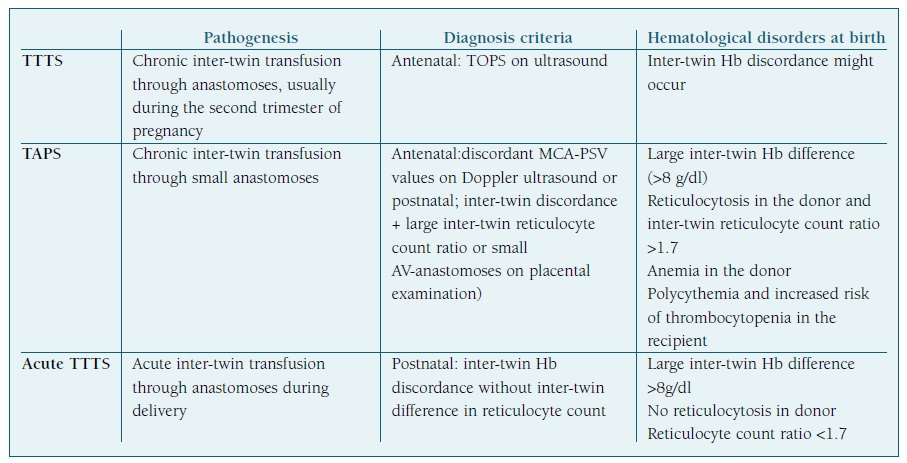
Hb - hemoglobin; TAPS - twin anemia-polycythemia sequence; TOPS - twin oligo-polyhydramnios sequence; TTTS - twin-to-twin transfusion syndrome.
Staging of TTTS and TAPS
TTTS has established staging, the so-called “Quintero Stages”, which divides the severity of TTTS into five stages. (5 The stages are described on Table II.
TABLE II Staging from TTTS and TAPS (prenatal - both old and new criteria - and postnatal) Adapted from “Twin-twin transfusion syndrome - What we have learned from clinical trials” by F.Djaafri et al, “Improved antenatal prediction of twin anemia-polycythemia sequence by delta middle cerebral artery peak systolic velocity: a new antenatal classification system” by L.Tollenaar et al, and “Twin Anemia Polycythemia Sequence: Current Views on Pathogenesis, Diagnostic Criteria, Perinatal Management, and Outcome.” by L.Tollenaar et al.
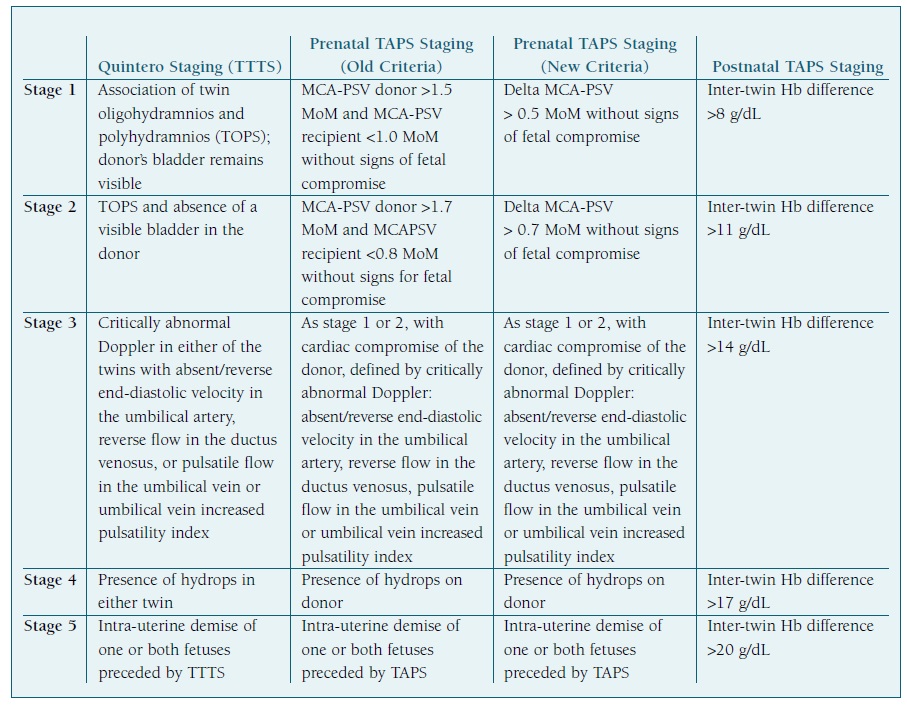
Hb - hemoglobin; MCA-PSV - middle cerebral artery peak systolic velocity; TAPS - twin anemia-polycythemia sequence; TOPS - oligohydramnios- polyhydramnios sequence; TTTS - twin-to-twin transfusion syndrome.
Staging the progression of TAPS is important for better research and clinical applicability concerning decision (and means) of treatments.
A prenatal and postnatal classification system of TAPS has been proposed4 and thus updated due to new prenatal diagnostic criteria based on delta MCA-PSV. (23 In these new prenatal classifications, stage 1 consists on a delta MCA-PSV >0.5 MoM, without further signs of fetal compromise. Stage 2 implies a delta MCA-PSV >0.7 MoM (new criteria), without further signs of fetal compromise. Stage 3 consists on the criteria from stage 1 or 2 with cardiac compromise of the donor, shown by critically abnormal Doppler: absent or reverse end-diastolic velocity in the umbilical artery, reverse flow in the ductus venosus, pulsatile flow in the umbilical vein or increased pulsatility index. Stage 4 is defined by the presence of hydrops on a TAPS donor. Stage 5 implies the demise of one or both fetuses, in a pregnancy previously known to be affected by TAPS. The postnatal staging of TAPS is based on the difference of hemoglobin values between the two fetuses (assuming that another criterion, such as the reticulocyte count ratio, is met for the diagnosis of TAPS to be made): an inter-twin hemoglobin difference >8 g/dL is the inferior limit for stage 1, >11 g/dL for stage 2, >14 g/dl for stage 3, 17 g/dl for stage 4 and 20 g/dl for stage 5. Whether these classifications offer an additional value to adequately stage and treat TAPS requires further investigation. (4
Table II summarizes staging of TTTS using the Quintero Stages, and prenatal (old and new criteria) and postnatal staging of TAPS.
Complications
Perinatal outcomes of TAPS vary from mild hematological complications to severe cerebral injury and perinatal death. (12
Cardiac decompensation of the donor, hydrops of donor and demise of any of the twins are complications stated in the staging classification of TAPS. (4
Neonates with TAPS have mainly short-term hematological complications. (27), (37 A blood transfusion is needed for most donors. (27 Recipients of TAPS may be severely polycythemic and require partial exchange transfusion (PET) often. (27
Thrombocytopenia is also common in TAPS. (27 Postnatal short-term renal dysfunction was also described38, and it was found to be more common in donors.
A study39 concerning fetal brain imaging following laser surgery for TTTS found out that the development of post-laser TAPS (as well as recurrence of TTTS) was a risk factor for prenatal brain damage. There is also a case report of a prenatal brain lesion in a spontaneous TAPS recipient, thought to be caused by hyperviscosity/polycythemia40. Bilateral deafness and spastic paralysis have also been reported. (41 The recipient of TAPS seems to be at greater risk for brain damage. (42
Prenatally acquired limb ischemia, including multiple limb ischemia, not explained by other factors has been reported in recipients of TAPS. (43
Prenatal management
There is no clear evidence on the optimal management of these pregnancies, and therefore it is suggested that management decisions should be made after careful individual analysis, including TAPS stage, gestational age, the feasibility and availability of the different types of intra-uterine intervention. (4
Laser surgery
Laser coagulation of the vascular anastomoses is the only curative treatment for TAPS. (4), (17 Moreover, laser therapy in TAPS can be technically more challenging than in TTTS17), (44 because of the possible absence of polyhydramnios and of a ‘stuck twin’, and difficult visualization of the very small communicating anastomoses. (20 When considering laser therapy for TAPS, preoperative amnioinfusion is thus advisable45. Laser surgery is also generally technically more difficult in anterior and centrally located placentas, due to limited access and visualization of the placental vascular bed10), (46 and in obesity46. Laser surgery is contraindicated with active labour, preterm premature rupture of membranes (PPROM), chorioamnion separation (CAS), active vaginal bleeding, or with subchorionic hematoma; short cervix and discordance for fetal malformations with TTTS are relative contraindications in certain centres. (10
A study17 evaluated the effectiveness of laser treatment on antenatally detected TAPS cases, compared to treatment by intrauterine transfusion or expectant management, and found no severe postnatal hematological complications and more time between diagnosis and birth. (17
The traditional technique for laser treatment is known as selective fetoscopic laser photocoagulation and consists of, during fetoscopy, tracing vessels from each fetus and visualizing where they end: vessels that leave the cord root as an artery (identified as so as the artery always crosses over the vein), disappear deeply into a cotyledon, and then exit returning to the same fetus as a vein are considered functional and not pathologic. (47 In contrast, a vessel that travels from one fetus, inserts into a cotyledon, and then appears to exit into the cord root of the other fetus is considered nonfunctional and pathologic, and are therefore photocoagulated. (47
Residual anastomoses after laser treatment are common. (48 Analysis of post-laser TAPS placentas typically show small diameter unidirectional AV anastomoses found within 2 cm of placental edge, and, due to the laser surgery, there are no remaining compensatory anastomoses that could alleviate the unbalanced blood transfer. (44
When performing the laser treatment it is thus also advisable to use the Solomon technique4), (44, developed to minimize these residual anastomoses49 and therefore the incidence of recurrent/persistent TTTS or postlaser TAPS. (47 Solomon technique consists in, after closing all the visible anastomoses with the selective technique47, coagulating a thin line of tissue at the placental surface from one edge of the placenta to the other47), (48 to close tiny anastomoses that might had not been visualised during the procedure. (48), (49
The Solomon trial reported less cases of TAPS and recurrent TTTS in the Solomon group compared to the control group, and it did not seem to be associated with an increase in any identifiable adverse effect or complications. (48 A systematic review50 including the Solomon trial and two other retrospective studies49), (51 concluded that the Solomon technique is certainly equivalent to the standard selective technique, has some advantages over it, with no increase in other adverse events, and is thus recommendable. Another review study52 also supported the use of the Solomon technique.
It is important to state that, based on these results, the Solomon procedure does not guarantee that recurrence of TTTS or TAPS do not occur, and therefore careful follow-up evaluation with serial MCA-PSV measurements, as well as measurements of amniotic fluid volumes, still remains crucial. (10), (48), (52
Moreover, if there was any previous laser treatment (as in, by definition, all cases of post-laser TAPS), careful consideration should be made before attempting a new laser procedure: if the anastomoses were not visualized in the first laser treatment for TTTS, it is unlikely that they will be identified any better during a second procedure. (45 However, changing the access route may be helpful. (45
Complications after placental laser surgery are common, with PPROM being the most frequent; post-laser CAS is a major risk factor for its occurrence. (10 Disruption of the amnion can result in pseudoamniotic constrictions on the distal extremities of one fetus, which is estimated to occur in 2% of post-laser pregnancies. (10
When laser treatment is not possible, other management options include expectant management, intrauterine blood transfusion (IUT) in the donor, with or without in-utero partial exchange transfusion (iPET) in the recipient, fetoscopic laser surgery, induction of labour/termination of pregnancy, and selective feticide. (4
Expectant management
Expectant management consists of close monitoring with ultrasound, including Doppler measurements of MCA-PSV. It can be considered in less severe cases of TAPS, such as stage 1-2. (4 This is also a valuable treatment option in presentations after 30 weeks’ gestation with a stable fetal condition. (17
Only one spontaneous regression of twin anemia-polycythemia sequence presenting in the first trimester was reported53. Some studies4), (54 mention another case in 2008 of an apparent spontaneous resolution of TAPS, probably due to thrombosis of the residual AV-anastomosis. The odds of spontaneous recovery are therefore low. (17
Intrauterine blood transfusion (IUT) with or without in-utero partial exchange transfusion (iPET)
Treatment with in-utero transfusions can be performed in the donor either intravascularly (in the umbilical vein of the anemic twin) or intraperitoneally. (4), (55 Intraperitoneal IUT is generally preferred, since it may allow slower absorption of red blood cells into the fetal circulation, preventing rapid loss of transfused blood in the circulation of the recipient twin. (4), (44), (46 This is especially important as a potential side effect of IUT treatment is worsening of the polycythemia hyperviscosity syndrome in the recipient. (4), (56
To reduce the risk of increasing polycythemia hyperviscosity, combining an IUT in the donor and iPET in the recipient can be useful. (4 iPET consists of, after taking a sample for the determination of hematocrit, taking some fetal blood from the umbilical vein of the recipient twin and then replace with 0.9% NaCl. (55 iPET for the recipient and IUT for donor are performed during the same procedural session, starting with the iPET55, and can also be done several times during the course of a pregnancy. (45), (55
However, it is important to state that IUT (with or without iPET) is only a symptomatic treatment for TAPS as it does not solve the underlying problem of small (residual) anastomoses4. IUT might be especially useful as a management option when laser treatment is not feasible, especially in cases of very early onset of TAPS (as a bridge management until laser surgery), placenta covering the whole anterior uterine wall, or obese patients. (46
Pregnancy termination/delivery
If the above management strategies are unsuccessful, further clinical progression then necessitates preterm delivery. (44 Indications towards delivery, taking into account the gestational age, include worrisome Doppler37, nonreassuring fetal heart rate19), (37 severe intrauterine growth restriction57, fetal hydrops41), (57, fetal cardiac failure41 or active labour. (58 In a literature review of 29 cases of TAPS, 23 of them were delivered by cesarean section. (19
Timing of cord clamping has importance on hemoglobin values at birth. (27 Delayed cord clamping could be beneficial to reduce the risk in anemia in donors, and early cord clamping advantageous to reduce the risks of hyperviscosity in recipients. (27 More research is needed on this topic.
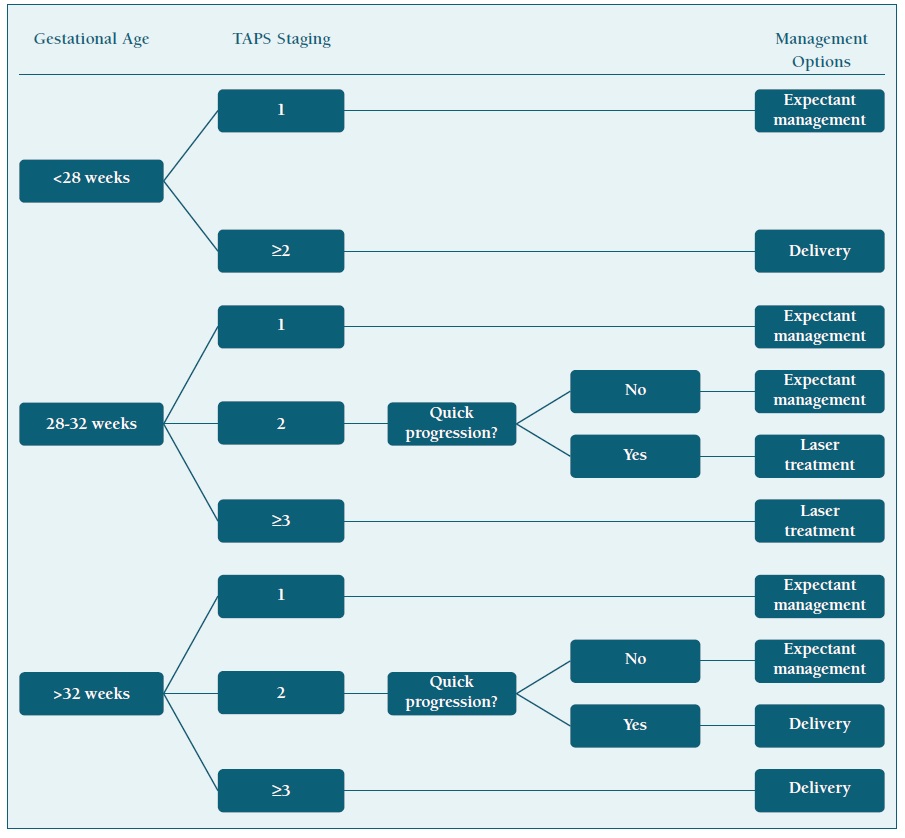
Management protocol
In the absence of a clear, evidence-based, antenatal management protocol, in a review study from the Leiden group (the most noticeable group on TAPS research), one proposal was made. According to this proposal, TAPS stage 1, and eventually stage 2, can be observed only with close monitoring by frequent ultrasound (including measurement of the MCA-PSV); if TAPS progresses quickly to stage 2 or in case of stage ≥3, active intervention should be considered. (4 Gestational age should been taken into account: below 28 weeks and laser treatment is feasible, laser treatment should be considered, as it is the only causal treatment for TAPS and it likely prolongs the pregnancy. (4), (17 When laser treatment is not feasible and gestational age is below 30-32 weeks of pregnancy, IUT should be considered as a management option; when repeated IUT transfusions are expected or in case of severe polycythemia in the recipient, PET of the recipient should also be made. (4 After 32 weeks, delivery is an acceptable management for case ≥3 or progressive stage 2 cases. (4
A simplified flowchart of this management protocol is shown on Table III.
Discussion and conclusions
TAPS is a pathology exclusive to monochorionic pregnancies, characterised by a striking difference of hemoglobin values between two fetuses: the anemic one (the donor) and the polycythemic (the recipient). TAPS happens due to chronic slow blood transfusion through the placenta from the donor to the recipient twin, probably due to small AV-anastomosis not enough compensated by the bidirectional AA anastomoses. This might happen spontaneously on the course of a monochorionic pregnancy, or after laser surgery for twin-to-twin transfusion syndrome.
TAPS is a newly described pathology, and that is reflected on the lack of consensual diagnostic parameters and the absence of a clear evidence-based management protocol. However, it is clear today that prenatal diagnosis of this condition relies on discrepant values of MCA-PSV between the fetuses. A delta >0.5 MoM between the twins’ MCA-PSV seems now to be the best prenatal diagnosis criterion. Absence of TOPS is an essential criterion for the prenatal diagnosis of TAPS. Postnatally, a hemoglobin difference >8 g/dl between the two newborns is essential for the diagnosis. Moreover, to differentiate TAPS from acute TTTS, other criteria should be met: a reticulocyte count ratio >1.7 or placental examinations revealing the presence of small AV anastomoses.
Screening of TAPS with MCA-PSV should be routine after laser surgery for TTTS. Screening for spontaneous TAPS in otherwise uncomplicated monochorionic twins is not so consensual, but growly recommended.
Prenatal and postnatal staging of TAPS has been proposed. Prenatal complications include prenatal brain injury, prenatal limb occlusion, fetal hydrops, cardiac decompensation and fetal death. Postnatal complications include persistence of prenatal existing injuries, complications related to prematurity and, specially, hematological complications caused by the anemia and polycythemia.
There is no clear evidence-based management algorithm for TAPS. Laser surgery is the only causal treatment for this condition, but it is not always possible, and it is technically more challenging in TAPS cases than in TTTS. The Solomon technique is recommended. Other prenatal management options include expectant management, intrauterine transfusion with or without further partial exchange transfusion, termination of pregnancy/delivery or selective feticide. Expectant management is common in less advanced cases of TAPS.
As a rare and newly described pathology, further research on TAPS is essential. To help this a web-based registry of TAPS cases (https://www.tapsregistry.org) was recently created.